- A Simple Synthetic Route of SiC Nanopowder Derived from Ultra-high Molecular Polycarbosilane
Sung Gun Bae*, Chang-Jin Park*, Dong-Geun Shin*†
* Aerospace Convergence Materials Center, Korea Institute of Ceramic Engineering & Technology, Jinju 52851, Republic of Korea
This article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Ultra-high molecular weight polycarbosilane (UHMW-PCS) was facilely synthesized through a catalytic process using a boron-based catalyst. Liquid silane, used as a starting material, was polymerized by adding B(OH)3 in an autoclave synthesis vessel, and polymerization was carried out effectively when the B(OH)3 content was 1 wt%. The synthesized UHMW-PCS was easily ground into fine powders of hundreds of nanometers in size through a ball mill, and SiC nanopowders were manufactured through direct heat treatment to 1200 and 1600°C without a curing step such as thermal oxidation. In this process, UHMW-PCS showed a high ceramic yield of over 92wt%, and the obtained SiC nanopowders showed a binary particle size distribution consisting of primary particles with a size of 50~100 nm and agglomerated secondary particles with a size of 300~100 nm. SiC nanopowders synthesized through this cost-effective process are expected to be applied to ultra-high temperature ceramic fiber composites or 3D printing SiC.
Keywords: Ultra-high molecular weight polycarbosilane, SiC nanopowders, Liquid silane
High-quality SiC powders have been investigated for specific uses such as ceramic fillers in SiC fiber matrix composites and ceramic 3D printing which are used in defense, aerospace and industrial heat exchanger applications [1-4], and the source materials for SiC single crystal growth in the physical vapor transport (PVT) process which are used in the high power semiconductor for hybrid vehicle (HV), power factor corrector (PFC), photovoltaic (PV) and train traction system applications [5,6]. Especially, SiC powders which are used for ceramic fillers in the SiC fiber matrix composites should be uniformly mixed with a polycarbosilane (PCS) solution, which is a well-known preceramic polymer of SiC ceramics for effective polymer infiltration and pyrolysis(PIP) process. However, it is difficult to obtain a stable infiltration solution because of the difference in their specific gravities [7]. For ceramic inks for 3D printing derived from preceramic polymer, the filler should have fine and uniform size distribution, and there must be no problem from UV light scattering issue. However, nano-sized SiC fine powder cannot overcome these problems because the color cannot avoid light scattering and shrinkage during thermal decomposition increases as the powder size decreases. If a powder-type high-molecular-weight PCS that does not only melt or desolve but also has a high ceramic yield is developed, it can be used as a filler material instead of SiC particles to improve some process issues such as the suspension stability of the precursor solution, the shrinkage, and the interaction at the interface between the filler and the matrix.
Generally, PCS is an inorganic polymer that has organic-like properties, e.g., dissolution, melting, and decomposition at high temperature. Therefore, a curing process is needed to prevent the collapse of a specific shape during heat treatment. In case of thermal oxidation, a typical curing method for PCS, unwanted oxygen should be introduced during the process and plays a role for the thermal decomposition at high temperature up to 1400°C in the final product. Several oxygen-free curing processes have been suggested such as electron beam radiation curing despite the higher cost of process [8]. On the other hand, Ishihara attempted to synthesize a fine SiC powder using high-molecular-weight PCS, which was separated by a supersaturation process, because the melting point of a polymer depends on its molecular weight [9]. However, it could not overcome to refrain from melting, and it was necessary to carry out a heat treatment after oxidation curing.
In this work, we have suggested a simple method for producing ultra-high molecular weight PCS (UHMW-PCS) using a boron catalyst. Traditional synthetic method, so-called yajima process, needed high temperature up to 450°C, high pressure up to 100 atm and relative longer process time. So, catalyst assisted process was adopted to overcome the issues in yajima process and make UHMW-PCS simply under mild synthetic conditions with relatively short process time. Furthermore, the formation of SiC nanopowders by organic-inorganic conversion and crystallization of UHMW-PCS powder without curing step were also investigated.
As a starting material for the synthesis of UHMW-PCS, liquid-type methyl silane (liquid silane) was used, which was prepared by the thermal breakdown of polydimthylsilane (PDMS) into short chain silanes.PDMS was heated to 400°C in an autoclave reactor, during which the decomposed silane was collected as liquid silane in an external receiver via a cooling tower. GC-MS was performed to determine the molecular structure of the methyl silane complex, and it was found that it mainly contained monosilanes containing 1 to 5 Si-Si bonds and some carbosilane with Si-C bonds.
100g of liquid silane (liquid-type methyl silane) was mixed with 1-5wt% boric acid (ACS reagent, ≥99.5%, Sigma-Aldrich) before closing a 500 ml-autoclave vessel and Ar gas was flowed before evacuation for removing oxygen in the vessel. Then, the autoclave vessel was heated to 300-400°C and held for 5-10 hours in an inert atmosphere. After the reaction has finished, a yellow-colored product was obtained at room temperature. Small amount of unreacted liquid silane was selectively separated away through a washing with toluene and filtration process. Then, remaining solid product was finely grinded using SiC ball-mill before drying in the oven, and a yellow-colored UHMW-PCS fine powder was obtained. It didn’t melt when heated and dissolved in any solvent.
10g of UHMW-PCS powder was loaded into a high temperature graphite furnace and directly heat-treated to 1200-1600°C for 1 hour without any curing step. Then, SiC fine powder was finally obtained at room temperature. The overall experimental process including the synthesis of UHMW-PCS and heat-treatment for the formation of SiC nanopowders is summarized in Fig. 1.
Fourier transform infrared (FT-IR) spectrum of UHMW-PCS was obtained using a FTS-175C spectrometer in the range 4000-400 cm-1. Attenuated total reflectance (ATR) and transparence method were used alternatively. ICP-AES (OPTIMA 4300, Perkin-Elmer, detection
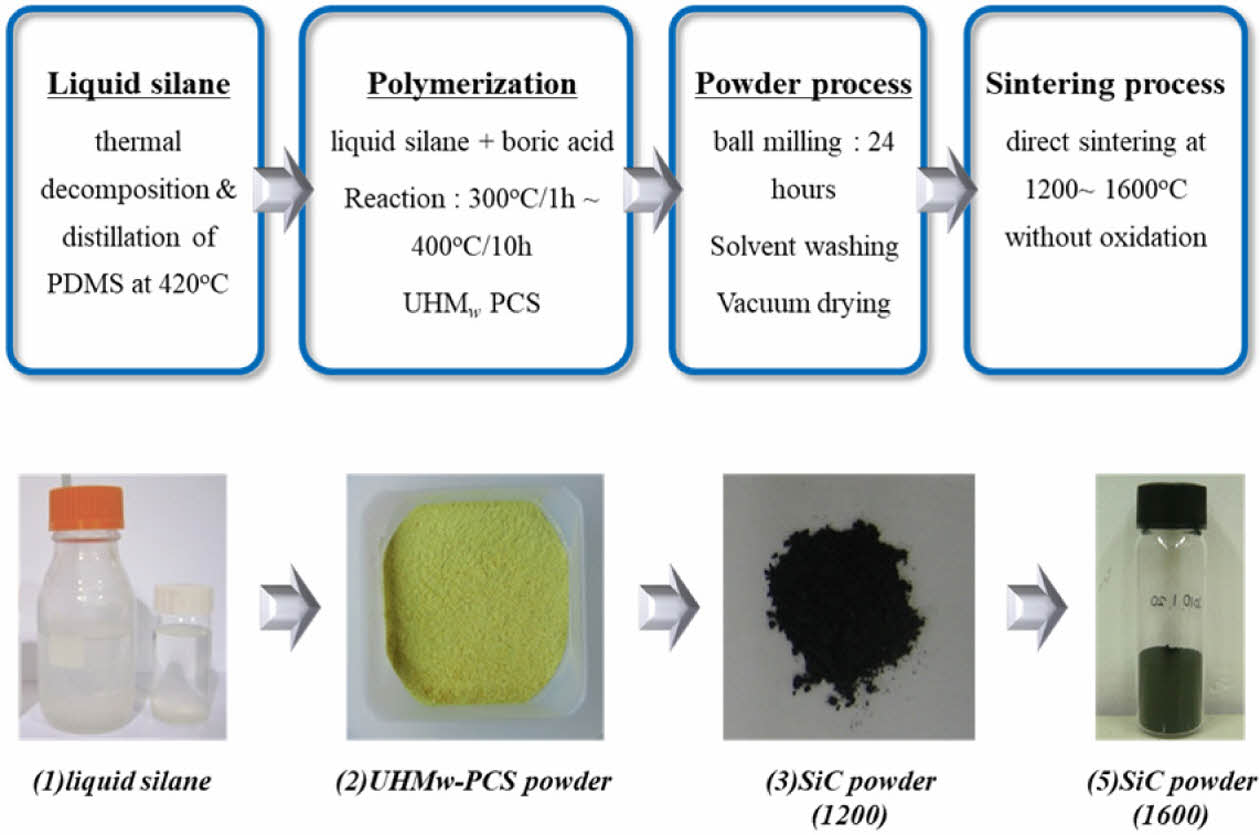
|
Fig. 1 Total experimental process for synthesis of UHMW-PCS and heat-treatment |
To maintain the quality of liquid silane, once PDMS was thermally decomposed, it was immediately collected in the external receiver from the reactor through a cooling tower to maintain a constant molecular weight distribution. Liquid silane as starting material was mainly contained 1~5 of linear silane monomers after thermal decomposition of polydimethylsilane as shown Fig. 2.
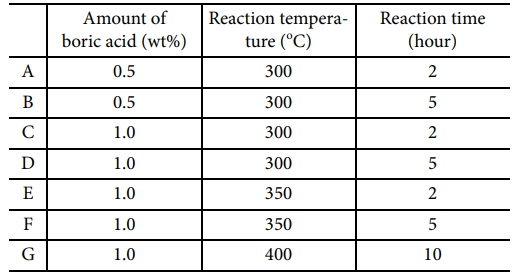
Since it is very difficult to polymerize liquid silane itself into a high molecular weight structure, boric acid, a strong acid catalyst, was added to promote the polymerization reaction [10,11]. Fig. 3 showed the molecular bonding characteristics and polymerization behavior of UHMW-PCS confirmed by fourier transform infrared spectroscopy (FT-IR), and the spectra of UHMW-PCS powder synthesized according to synthesis temperature, time and the amount of boric acid was compared with liquid silane and PDMS. Synthesis conditions were also listed in the Table 1.
After PDMS was thermally decomposed, several new IR absorption peaks appeared, the vibration of the -Si-C- backbone of PCS was observed at 1360 and 1020 cm-1, while the stretching vibration of Si-H and the deformation of Si-CH3 were observed at 2100 and 1410 cm-1 [12,13]. A trace amount of carbosilane monomer that has Si-C bond in their backbone was also present in the liquid silane, and a weak peak was observed at 1360 cm-1. This means that the PDMS was simply pyrolyzed into short chains and the conversion of backbone structure into -Si-C- was negligible. In other words, it was possible to promote a uniform catalytic reaction by converting PDMS in the form of a fine powder into liquid silane. Weak peak continued to get stronger depending on the synthesis conditions and was strongest for sample G conditions.
The stretching vibrations of Si-H and the deformation of Si-CH3 became weaker with increasing reaction conditions (see Table 1). Other peaks also provide the same information, indicating an increasing degree of polymerization of the carbosilane structure [14]. In the FT-IR spectrum of UHMW-PCS corresponded to that of commercial PCS [15]. The specific broad peaks at 2100 and 1400 cm-1 in the UHMW-PCS spectrum represented the high degree of polymerization [14,15]. Therefore, it was confirmed that UHMW-PCS was successfully polymerized during the autoclave reaction. However, it’s very hard to analyze the molecular weight of UHMW-PCS because it didn’t dissolve in any solvent and was impossible to measure Mw using gel-permeation chromatography (GPC). There are several acidic catalysts reported for synthesis of PCS such as halide catalysts, boron catalysts, zeolite(ZSM-5) and g-Al2O3 [11,12,16,17] and among them, boric acid is most strong solid acid catalyst which is suitable for effective polymerization.
A thermogravimetry analysis was carried out to compare the thermal decomposition behavior of as-synthesized UHMW-PCS prepared at various synthetic conditions as shown in Fig. 4. The thermal behavior varied significantly depending on the synthesis temperature, time and the amount of boric acid.
In the sample A-D which were synthesized at 300°C, thermal decomposition behavior was very complicated because of insufficient synthesis. However, ceramic yield was constantly increased with synthetic condition. When the amount of boric acid was fixed to 1.0wt%, the ceramic yield critically increased with reaction temperature and time and it reached up to 92wt% at the synthetic condition of the sample G. There was still a small amount of unreacted silane remaining and it was easily removed by dissolving in a toluene.
In case of the commercial PCS, weight decreased between 300 and 600°C owing to the decomposition of the organic parts of PCS into CH4, C2H6, H2, or other minor gases [18,19] and the evaporation of the lower Mw portions. Then, approximately 68% of the weight remained as residue after pyrolysis was complete. The DTG curve of the commercial PCS also showed a three major weight reduction at around 430°C, 550°C, and above 600°C related to evolution of decomposed gases during heat-treatment. On the other hand, when synthesized above 350°C, only two weight changes were observed.350°C. First weight change was related to the previously mentioned unreacted residual silane, and second weight change was related to the conversion reaction into inorganic product. For sample G, the organic parts were decomposed at around 600-800°C and the ceramic yield was 92.6wt% ceramic yield. This is closely related to the hydrogen evolution but not to the CH4 gas that might have been detected around 600°C.
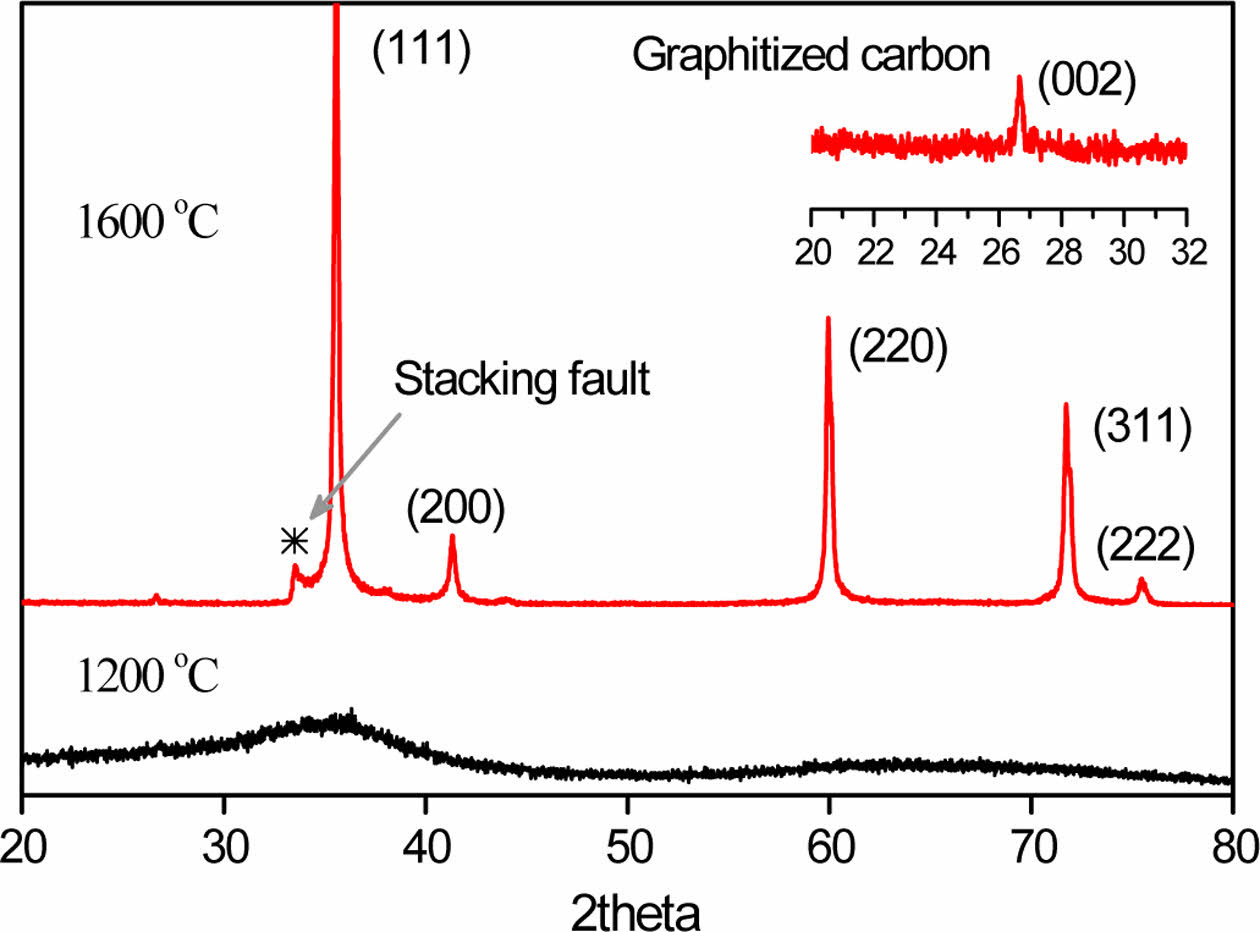
UHMW-PCS powder was heat-treated at 1200 and 1600°C for 1 h in a graphite furnace without curing step. Generally, PCS was boiled up and lost its initial shape when it was heated up without curing step such as thermal oxidation. In case of UHMW-PCS powder was held its powder shape during the heat treatment, and it was successfully pyrolyzed and converted into SiC crystals. Crystallization of the sample was confirmed by x-ray diffraction and is presented in Fig. 5.
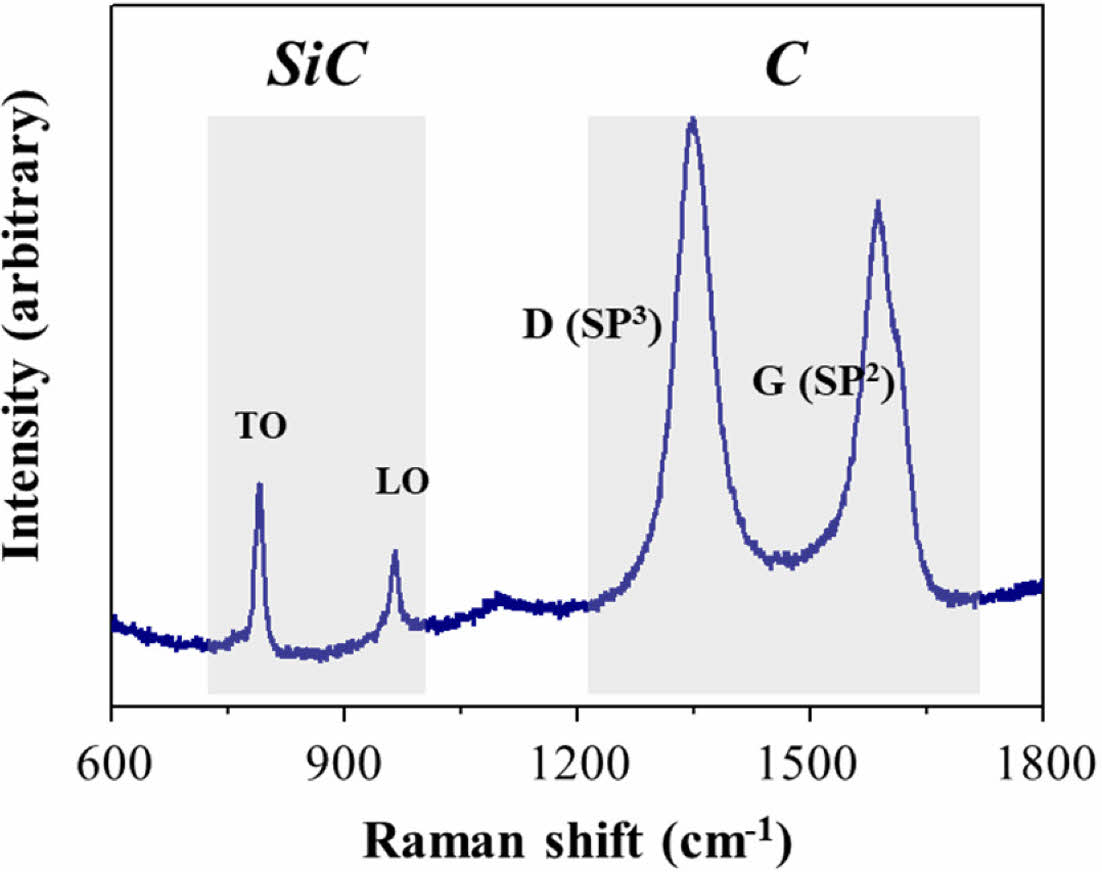
From the diffraction peaks, no distinctive peaks were observed except only one broad peak related to (111) β-SiC at around 35° at 1200°C. On the other hand, when the heat-treatment temperature increased to 1600°C, sharp peaks appeared. The diffraction peaks correspond to the β phase of the SiC (3C-SiC) structure, except for the peak at 33.5°. In general, the 3C-SiC polytype contains stacking faults and twins in the {111} planes to decrease the crystal formation energy [8,18,20,21]. The other weak peak was also observed at 2q=26.5° although it is too small to find out. This peak was assumed to be related to nano-crystalline carbon phase [20,22]. The state of SiC can be confirmed through Raman analysis, and the Raman spectrum of SiC prepared at 1600°C is presented in Fig. 6. The transverse optic (TO) phonon and a longitudinal optic phonon (LO) modes of SiC were detected at Raman shifts of 790 and 965 cm-1, which indicates that the SiC crystals were well grown. Moreover, the Raman spectrum shows two strong and clear peaks at 1600 and 1400 cm-1, which are assigned to the D and G bands of graphitized carbon. It is a common behavior of polymer-derived ceramics that excess carbon is left with graphitization through heat-treatment [23,24], and UHMW-PCS was no exception.
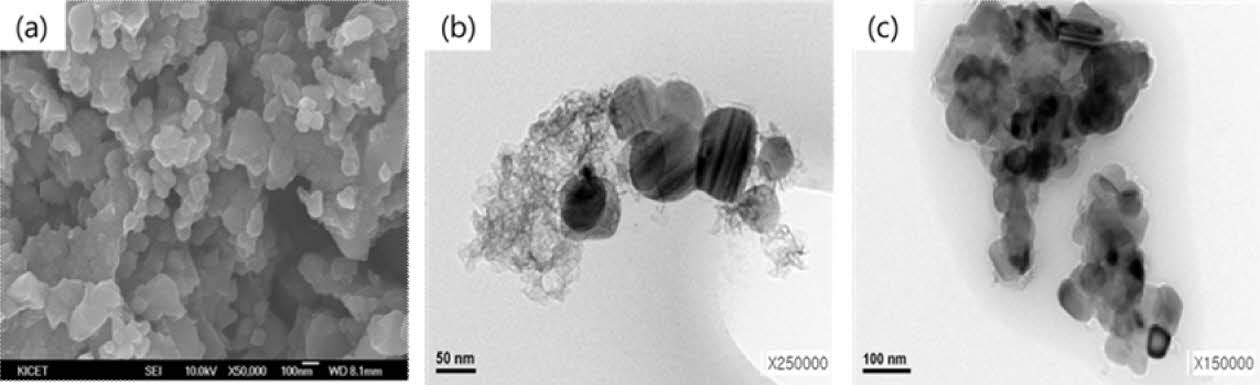
In this figure, the D band was stronger than G band and it shows that carbon exists in the form of glassy or nanocrystalline structures. The presence of oxygen or other impurities makes hidden bands between D and G bands which means that there were no other impurities except graphitic carbon. The tiny peak at 2q=26.5° in the x-ray diffraction pattern in Fig. 5 also corresponds to graphitized carbon [25,26]. Graphitized carbon was also observed among SiC particles in a transmission electron microscopy (TEM) image. Fig. 7 showed (a) High magnificent FE-SEM and (b, c) TEM images of a SiC nanopowders prepared at 1600°C.
SiC particles are well crystallized when heat-treated at 1600°C and their crystal sizes were in the range of 50-100 nm. Some were agglomerated together to make secondary particles around 300-500 nm. It looks like a scrap of paper and easily distinguished with SiC particles.
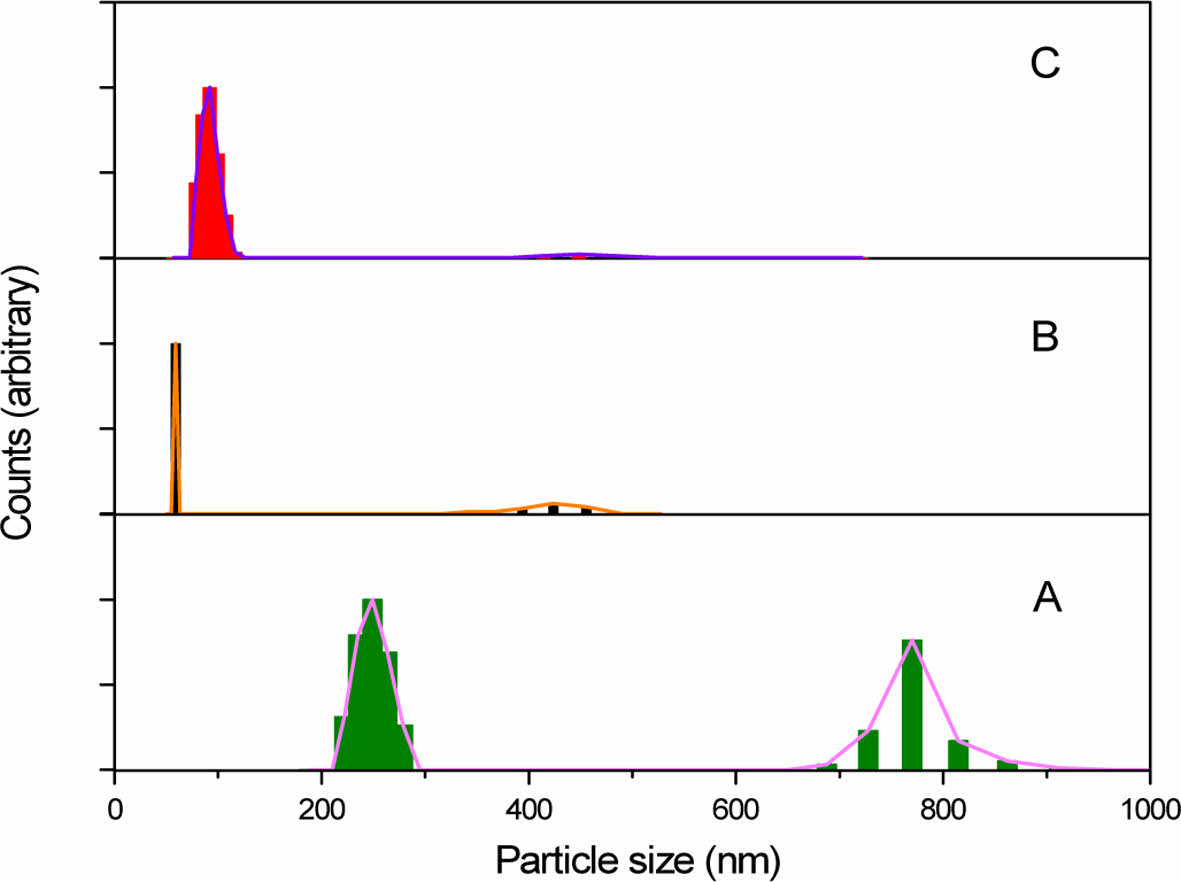
The growth behavior of particles was also verified by a particle-size analysis, and the results are shown in Fig. 8. The particle-size distribution of UHMW-PCS, which was prepared by ball milling to be used as a precursor for SiC, was measured to be bimodal in the ranges of 200-300 nm and 650-900 nm. A larger portion of particles, which were presumed to be physically aggregated, did not affect the size of SiC products because the area of the larger size decreased after heat-treatment.
When UHMW-PCS powder was heated to 1200°C, the primary particle sizes dramatically decreased during the thermal decomposition and nucleation of SiC. Then, it slightly increased again to around 100 nm at 1600°C by crystal growth, as confirmed by the x-ray diffraction pattern. As a result, a SiC nanopowders with a very uniform particle size was obtained using UHMW-PCS as a SiC precursor. This procedure is very simple and cost-effective, and there was no need for a precursor powder to stabilize by thermal oxidation or other additional curing step, which causes oxygen impurities.
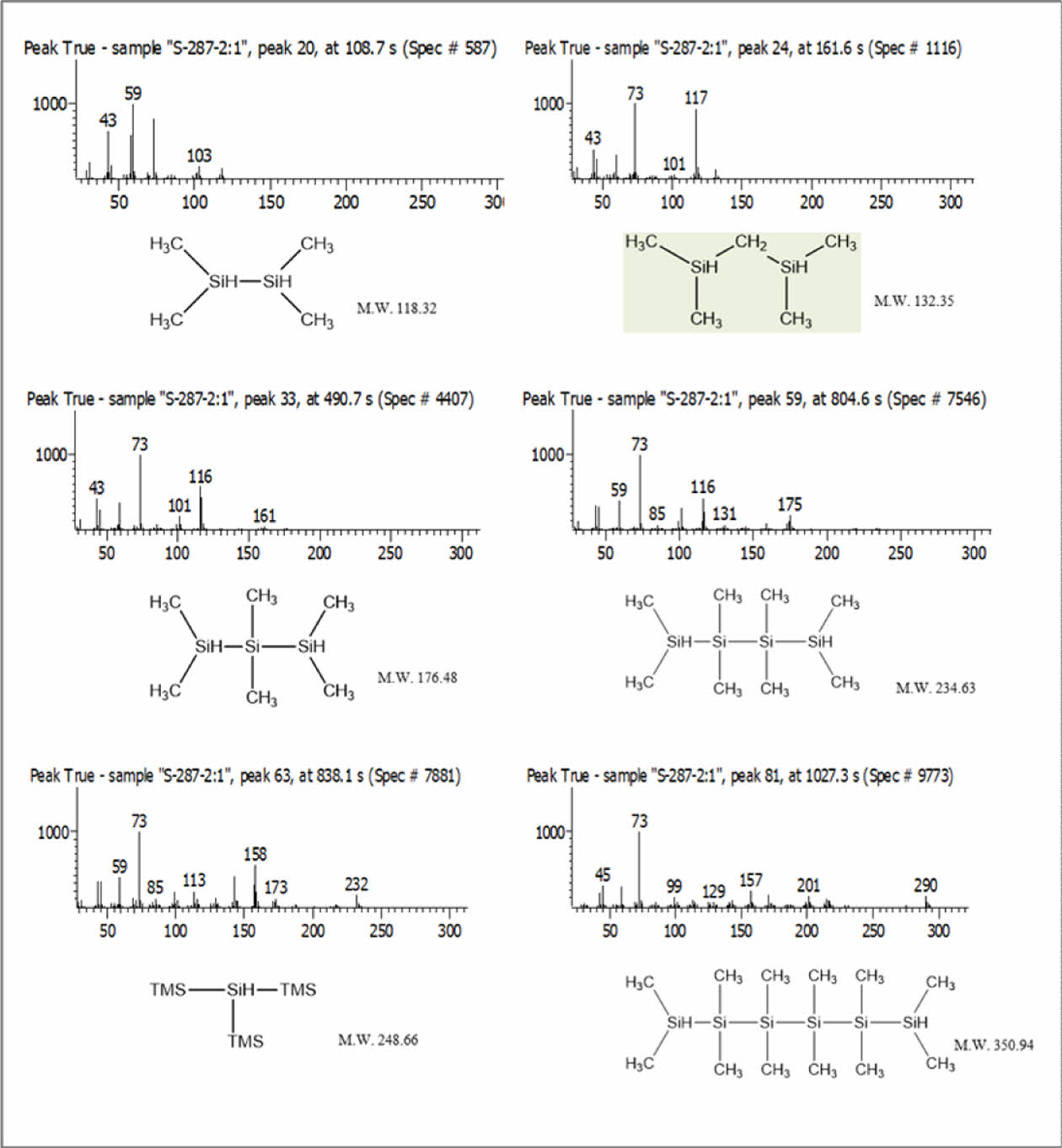
|
Fig. 2 Molecular structure of liquid silane from GC-MS results |
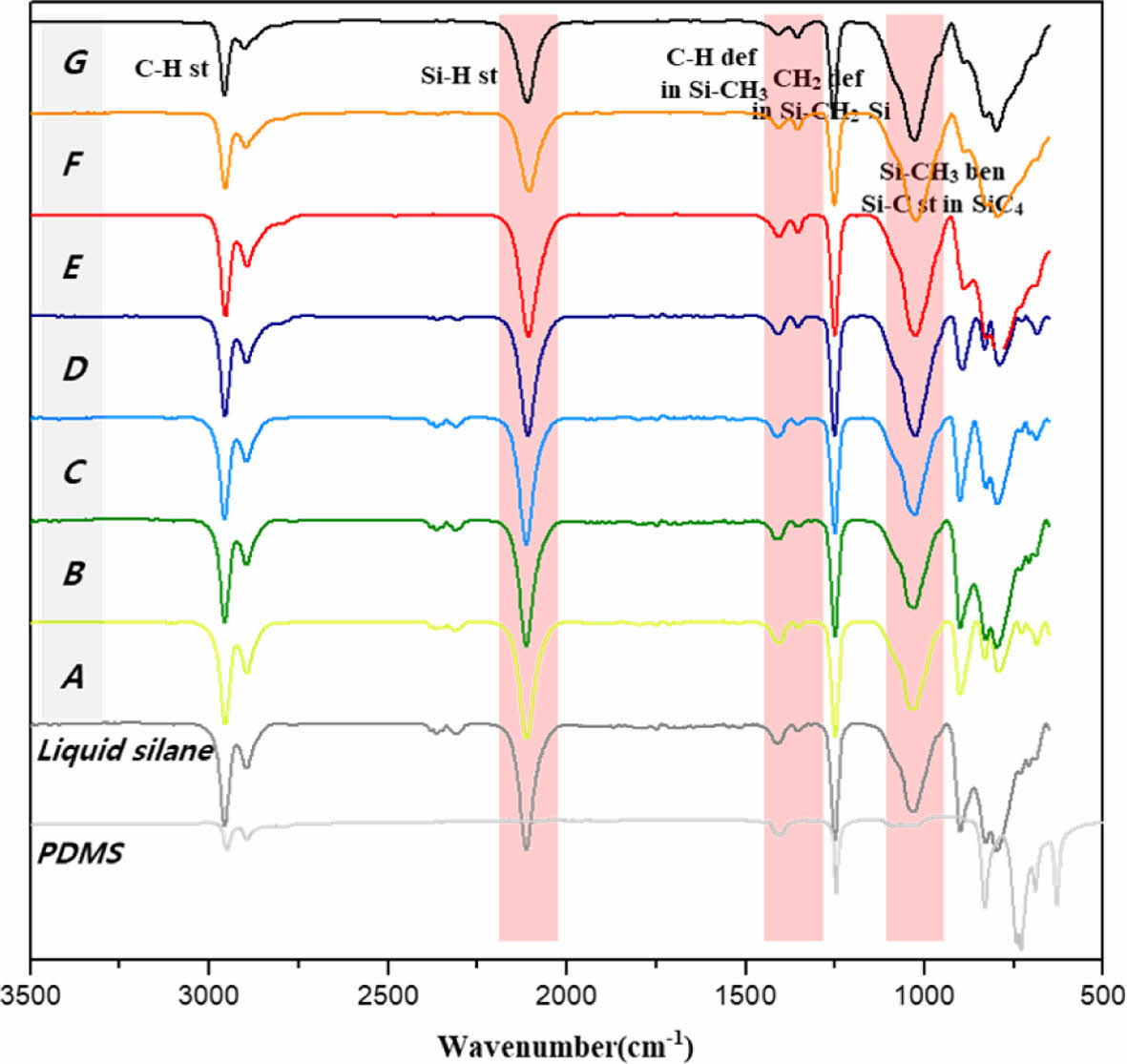
|
Fig. 3 FT-IR spectra of UHMW-PCS powder synthesized depending on the synthesis temperature, time and the amount of boric acid (sample A-G) compared with liquid silane and PDMS |
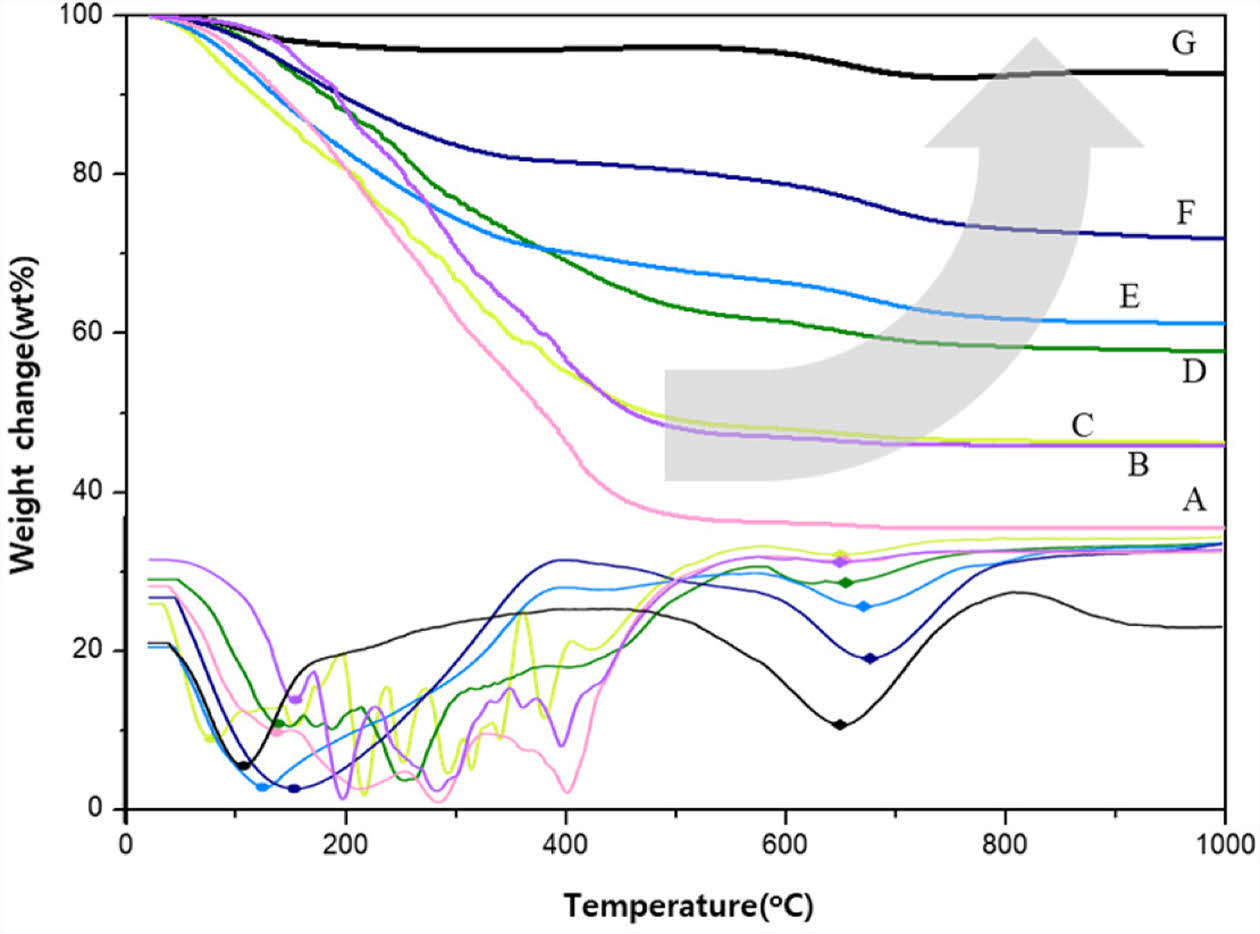
|
Fig. 4 Thermogravimetry analysis of as-synthesized UHMW-PCS prepared at various synthetic conditions as listed Table 1 |
|
Fig. 5 X-ray diffraction patterns of SiC powders obtained by the heat treatment of UHMW-PCS at 1200 and 1600°C |
|
Fig. 6 Raman Spectrum of SiC powder obtained by the heat treatment of UHMW-PCS at 1600°C |
|
Fig. 7 . (a) High magnificent FE-SEM and (b, c) TEM images of a SiC nanopowder prepared at 1600°C |
|
Fig. 8 Particle-size distributions of the (A) UHMW-PCS powder and SiC powders prepared at (B) 1200 and (C) 1600°C |
|
Table 1 Experimental conditions of as-synthesized UHMW-PCS prepared at various synthetic conditions |
SiC nanopowders were successfully fabricated using UHMW-PCS obtained by catalytic reaction of liquid silane derived from PDMS using boric acid. When 1.0wt% of boric acid was assisted to the synthesis of UHMW-PCS, liquid silane was effectively polymerized and then it was decomposed and crystallized to SiC without any curing step such as thermal oxidation. It reached up to 92wt% at the synthetic condition of the sample G. The size of SiC particles were in the range of 50-100 nm including the secondary particles around 300-500 nm.
The cost effective SiC nanopowders prepared by a simple synthetic route are expected to be promising candidates for high quality ceramic fillers for the matrix in high temperature SiC fiber composites and sintered SiC, 3D printing SiC for aerospace, defense industries.
UHMW-PCS : ultra-high molecular weight polycarbosilane, SiC: silicon carbide, PIP : polymer infiltration and pyrolysis, PVT: physical vapor transport, HV: hybrid vehicle, PDMS: polydimethylsilane, XRD: x-ray diffraction, TGA: thermos-gravimetric analysis, FE-SEM: field-emission scanning electron microscopy, XPS: x-ray photoelectron spectroscopy, TPP: two-point probe.
This research was financially supported by the Institute of Civil Military Technology Cooperation funded by the Defense Acquisition Program Administration and Ministry of Trade, Industry and Energy of Korean government under grant No.21-CM-CE-03.
- 1. He, R., Fang, D., Wang, P., Zhang, X., and Zhang, R., “Electrical Properties of ZrB2-SiC Ceramics with Potential for Heating Element Applications,” Cermaic International, Vol. 40, No. 7, 2014, pp. 9549-9553.
-

- 2. Yoon, B., Lee, S.H., Lee, H.S., Hwang, G., and Kim, B., “Effect of the Si-C Powder Prepared by Mechanical Alloying on the Densification of Silicon Carbide Powder,” Journal of the Korean Ceramic Society, Vol. 53, No. 1, 2016, pp. 99-104.
- 3. Tang, Z., Yi, M., Zhou, Y., Liu, R., and Peng, K., “Effect of High-temperature Heat Treatment on the Microstructure and Mechanical Behavior of PIP-based C/C-SiC Composites with SiC Filler,” Journal of the European Ceramic Society, Vol. 41, No. 15, 2021, pp. 7610-7619.
-

- 4. Masaki, K., Yoshikazu, M., Akinori, Y., Shinji, O., Tadashi, I., Hauyoshi, K., Yukai, Y., Yoshio, T., Keigo, E., and Takao, N., “Evaluation of Spaceborne SiC Mirror Materials Using Samples Cut from the Periphery of a Mirror Body,” Journal of Materials Engineering and Performance, Vol. 23, 2014, pp. 850-858.
-

- 5. Shin, D.G., Kim, B.S., Son, H.R., and Kim, M.S., “Study on the growth of 4H-SiC Single Crystal with High Purity SiC Fine Powder,” Journal of the Korean Crystal Growth and Crystal Technology, Vol. 29, No. 6, 2019, pp. 383-388.
-

- 6. Noboru, O., Tatsuo, F., Masakazu, K., and Hirokasu, Y., “Growth of Large High-quality SiC Single Crystals,” Journal of Crystal Growth, Vol. 237-239, part 2, 2002, pp. 1180-1186.
-

- 7. Qiance, Z., Han, L., Tianlu, Q., Philip, J.W., and Ping, X., “Simple and Efficient Densification of SiCf/SiC Composites by Gradated Concentration Polymer Infiltration and Pyrolysis,” Materials Science and Engineering: A, Vol. 874, 2023, pp. 2023-2031.
-

- 8. Ichikawa, H., “Development of High Performance SiC Fibers Derived from Polycarbosilane Using Electron Beam Irradiation Curing-A Review,” Journal of the Ceramic Society of Japan, Vol. 114, Issue 1330, 2006, pp. 455-460.
-

- 9. Ishihara, S., Nishimura, T., and Tanaka, H., “Precipitation Processing to Synthesize Fine Polycarbosilane Particles for Precursors of Silicon Carbide Powders,” Journal of the Ceramic Society of Japan, Vol. 114, Issue 1330, 2006, pp. 507-510.
-

- 10. Yajima, S., Hayashi, J., and Omori, M., “Synthesis and Characterization of Novel Preceramic Polymer for SiC,” Chemistry Letter, Vol. 4, No. 9, 1975, pp. 931-934.
-

- 11. Hasegawa, Y., Kobori, T., and Fukuda, K., “Organosilicon Polymer and Process for Production, US Pat. No. 4,590,253, 1986.
-

- 12. Shin, D.G., Riu, D.H., Kim, Y., Kim, H.R., Park, H.S., and Kim, H.E., “Characterization of SiC Fiber Derived from Polycarbosilanes with Controlled Molecular Weight,” Journal of Korean Ceramic Society, Vol. 42, No. 8, 2005, pp. 593-598.
-

- 13. Lee, Y.J., Lee, J.H., Kim, S.R., Kwon, W.T., and Klepeis, J.H.P., “Synthesis and Characterization of Novel Preceramic Polymer for SiC,” Journal of Materials Science, Vol. 45, 2010, pp. 1025-1031
-

- 14. Schilling Jr, C.L., Wesson, J.P., and Williams, T.C., “Polycarbosilane Precursors for Silicon Carbide,” Journal of Polymer Science: Polymer Symposia, Vol. 70, No. 1, 1983, pp. 121-128.
-

- 15. Sawai, Y., Iwamoto, Y., Okuzaki, S., Yasutomi, Y., Kikuta, K., and Hirano, S.I., “Synthesis of Silicon Carbide Ceramics Using Chemically Modified Polycarbosilanes as a Compaction Binder,” Journal of Materials Science, Vol. 82, No. 8, 2004, pp. 2121-2125.
-

- 16. Kim, Y., Shin, D.G., Kim, H.R., and Riu, D.H., “Preparation of Polycarbosilane Using a Catalytic Process and Its Practical Uses),” Key Engineering Materials, Vol. 287, 2005, pp. 108-111.
-

- 17. Huh, S.H., Shin, D.G., and Riu, D.H., “A Simple Synthetic Route of Polycarbosilane Precursor Using Nanoporous Anodized Aluminum Oxid,” Catalytic Communities, Vol. 10, Issue 2, 2008, pp. 208-212.
-

- 18. Shin, D.G., Cho, K.Y., and Riu, D.H., “Fabrication of Nanoporous Silicon Carbide Fibres by Thermal Treatment,” Advances in Applied Ceramics, Vol. 113, 2014, pp. 2121-2125.
-

- 19. Takeda, M., Imai, Y., and Ichikawa, H., “Thermal Stability of the Low Oxygen Silicon Carbide Fibers Derived from Polycarbosilane,” 16th Ann. Conf. Comp. & Adv. Ceram. Mater. Part1., 1994, https://doi.org/10.1002/9780470313954.ch23.
-

- 20. Le Coustumer, P., Monthioux, M., and Oberlin, A., “Understanding Nicalon¢ç Fiber,” Journal of European Ceramic Society, Vol. 11, No. 2, 1993, pp. 95-103
-

- 21. Porte, L., and Sartre, A., “Evidence for a Silicon Oxycarbide Phase in the Nicalon Silicon Carbide Fiber,” Journal of Materials Science, Vol. 24, No. 1, 1989, pp. 271-275.
-

- 22. Sadezky, A., Muckenhuber, H., Grothe, H., Niessner, R., and Pöschl, U., “Raman Microspectroscopy of Soot and Related Carbonaceous Materials: Spectral Analysis and Structural Information,” Carbon, Vol. 43, No. 8, 2005, pp. 1731-1742.
-

- 23. Sasaki, Y., Nishina, Y., Sato, M., and Okamura, K., “Raman Study of SiC Fibers Made from Polycarbosilane,” Journal of Materials Science, Vol. 22, No. 2, 1987, pp. 443-448.
-

- 24. Karlin, S., and Colomban, P., “Raman Study of the Chemical and Thermal Degradation of As-received and Sol-gel Embedded Nicalon and Hi‐Nicalon SiC Fibers Used in Ceramic Matrix Composites,” Journal of Raman Spectroscopy, Vol. 28, No. 4, 1997, pp. 219-228.
-

- 25. Chollon, G., Pailler, R., Canet, R., and Delhaes, P., “Correlation between Microstructure and Electrical Properties of SiC-based Fibers Derived from Organosilicon Precursors,” Journal of European Ceramic Society, Vol. 18, No. 6, 1998, pp. 725-733.
-

- 26. Cao, S., Wang, J., and Wang, H., “Formation Mechanism of Large SiC Grains on SiC Fiber Surfaces during Heat Treatment,” CrystEngComm, Vol. 18, No. 20, 2016, pp. 3674-3682.
-

 This Article
This Article
-
2025; 38(1): 1-6
Published on Feb 28, 2025
- 10.7234/composres.2025.38.1.001
- Received on Dec 17, 2024
- Revised on Jan 4, 2025
- Accepted on Jan 15, 2025
 Services
Services
- Abstract
1. introduction
2. experiments
3. results and discussion
4. conclusions
abbreviations
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Dong-Geun Shin
-
Aerospace Convergence Materials Center, Korea Institute of Ceramic Engineering & Technology, Jinju 52851, Republic of Korea
- E-mail: dgshin73@kicet.re.kr






 Copyright ⓒ The Korean Society for Composite Materials. All rights reserved.
Copyright ⓒ The Korean Society for Composite Materials. All rights reserved.