- Comparative Study of Metal-Polyphenol Network Coating and Plasma Treatment for Surface Modification of Thin Film Membranes
Khin Moe Lwin*, Pyone Pyone Chit*, Nur Elis Sharmila Binti Zulazmi**, Jinseung Bae*, Sungsu Park*, Kyunghoon Kim*†
* School of Mechanical Engineering, Sungkyunkwan University, Suwon 16419, Republic of Korea
** Department of Global Smart City, Sungkyunkwan University, Suwon 16419, Republic of KoreaThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Water is one of the important natural resources on earth whereas only 3% of the water is fresh and suitable for drinking. Membrane technology offers efficient and versatile solutions for various water treatment challenges, ranging from filtration and separation to desalination and water reuse. However, membrane fouling caused by contaminants remains a significant issue that impairs performance. Enhancing the surface hydrophilicity of membranes mitigates fouling and answers the recovery of membrane life span. Here, the two post-treatment technologies for surface hydrophilicity enhancement: surface coating and plasma treatment, were investigated, and compared the enhancement behavior originating from two. Several parameters for enhancing hydrophilicity such as surface morphologies, surface roughness, and bulk properties varied by two treatment methods are reported. As a result, it indicated an increase of surface hydrophilicity (i.e., from >35% to 60%) for both technical approaches, in which the surface coating technology offered superior wetting dynamics, revealing the absence of hydrophobic surface irregularities over the membrane surface.
Keywords: Hydrophilicity, Surface-coating, Surface modification, Plasma-treatment, Wetting
Thin film membranes are being widely utilized in water treatment applications by offering ease of modification, simple fabrication techniques, diverse selectivity, and separation efficiency [1]. Several parameters govern the performance of membranes ranging from surface roughness, surface hydrophilicity, permeability, pore size distribution, and mechanical properties. Meanwhile, applying post-treatment surface modification technologies is the impactful answer as the membrane surface directly reflects the water contact and fouling resistance. Various surface modification methods have been suggested to enhance the surface properties of membranes. These modifications include physical modifications such as surface-coating [2], blending, and chemical modifications such as polymer functionalization, grafting [3], and plasma treatment [4].
Surface coating enables the deposition of an active layer over the surface which can go attractions or rejections to the foreign colloids. Several works suggest the reproducibility of membrane coating technology using polymer [5], and nanomaterials [6,7] for effective water treatment applications. The low-pressure plasma treatment can introduce polar groups on the topmost surface of the membrane to enhance hydrophilicity or can generate the active species to initiate polymer grafting without affecting the bulk properties of polymer [4,8]. Various kinds of polymer membranes such as Polyethersulfone (PES), Polyvinylidene fluoride (PVDF), Polyvinyl chloride (PVC), polysulfone, Polyacrylonitrile (PAN), and Poly(propylene) have been investigated by plasma treatment and showed great potentials to improve the antifouling and permeability characteristics of the membranes. These treated plasma membranes have been applied to various microfiltration [9], ultrafiltration (UF) [10], nanofiltration (NF), and gas separation (GS) membranes.
Among the various parameters that influence the membrane performance, one frontier is enhancing the hydrophilicity of the membrane surface as it can significantly reflect the wettability answering the better permeability for the improved flow rates [11]. Additionally, hydrophilic membranes can exhibit reduced fouling tendencies with the hydrophilic/hydrophobic rejection with the organic collides from the water source leading to the facilitated rejection and longer membrane life span [12].
For enhancing hydrophilicity, herein, metal-polyphenol network (MPN) coating which is a known self-healing and adhesive material was applied [13]. The Fe ions and tannic acid (TA) were chosen to undergo the metal polyphenol complex, and both are regarded as non-toxic and abundant materials in nature. Furthermore, the MPN network is composed of several hydrophilic (-OH) hydroxyl groups and Fe-O coordination resulted from the polyphenols. On the other hand, the chemical nature of the plasma gases and treatment time have a strong influence on the surface modification of the membrane. Many different gases and vapors are used as process gases including air [14-16], oxygen [17-19], nitrogen [20], argon [21-23], helium [24], ammonia [25], carbon dioxide [26], etc. Among them, the vacuum oxygen plasma treatment is very effective in enhancing the hydrophilicity of the membrane due to the formation of functional groups (hydroxyl, carbonyl, and carboxyl groups) [9].
In this study, we investigated the influence of MPN coating and oxygen plasma treatment on the membrane morphology, surface roughness, water contact angles, and the pore structures of the membranes. On top of that, we also explored the wetting behaviors that emerged from these two routes. The result suggests that both types of modified TFMs give the possibilities for the applications in filtration of various types of foulants such as protein and natural organic matter (NOM), oil-water separation, and bacterial virus separation due to their cost effectiveness and versatility.
2.1 Materials
Polysulfone (Psf), polyvinylpyrrolidone (PVP), Tannic acid (TA), Iron (III) chloride (FeCl3) were purchased from Sigma-Aldrich (USA). The solvent for membrane preparation N-methyl-2-pyrrolidone (NMP, 99.7%) was purchased from Daejung Chemical Co (Korea).
2.2 Fabrication of Thin Film Membranes (TFM)
The polysulfone (Psf) membranes were fabricated using the non-solvent-induced phase separation method (NIPS). A solution containing 13.5 wt% Psf and 0.5 wt% PVP additive dissolved in NMP was prepared. To ensure a uniformly dissolved casting solution, the mixture was heated on a hot plate at 70oC while gently stirring at 100 RPM to prevent the formation of bubbles. Subsequently, the clear viscous casting solution was cooled to room temperature and stand still to make sure there were no bubbles remaining inside. Then, the polymer solution was casted onto a flat glass plate using a casting knife which was adjusted to 150 µm. Then, it was immediately immersed in a water bath filled with tap water, where the TFM was formed through phase inversion. The membrane was left in the DI water bath for more than 25 hr to remove any residual solvents left. Fig. 1
2.3 Surface modification of TFMs by Metal polyphenol coating
The MPN coating on the UF membrane was carried out by the surface interaction at the active layer interface of TA/Fe3+ as presented in Fig. 2. Tannic acid and FeCl3+ were dissolved in DI water with different concentrations for thin and thick coatings of MPN (Table 1). Metal polyphenol was allowed for the complex formation by dipping the membrane inside the TA solution and then in FeCl3+ solution. After all, the MPN-coated membranes were washed with DI water to remove non-incorporated or excessive MPN particles over the membrane.
2.4 Surface modification of TFMs by Plasma Treatment
Polysulfone membrane (TFM) sheets go through the vacuum oxygen plasma treatment Femto-science CUTE Plasma System for surface modification. The flow rate was set to 20 standard cubic centimeters per minute (sccm), pressure 0.3-0.9 Torr, and power 60 W. Polysulfone films were treated with various plasma treatment times of 20, 40, 60, and 120 s. The step-by-step plasma treatment was presented in Fig. 3.
2.5 Characterization of TFMs
The morphology of UF membranes and coated surfaces were imaged by FE-SEM (JSM-7600F, JEOL). The membranes were dried in ambient conditions for surface image and freeze-fractured in a liquid nitrogen bath for surface imaging. For FT-IR ATR analysis, the samples were freeze-dried and prepared by placing an active layer surface on the ATR crystal. The AFM imaging of all fabricated membranes was acquired by Park Systems XE7 AFM instrument. The AFM images were taken by tapping mode using the commercial silicon cantilever. The contact angle of the membrane surface was measured to investigate the hydrophilicity of the membrane using a contact angle analyzer (Pheonix-MT(M), S-EO). In the sessile drop method, each contact angle was measured immediately after dropping ~3 μl of DI water. In order to determine the effect of the hydrophilic behavior of polymer membranes with plasma treatment, the as-prepared TFM and plasma-treated TFMs were observed through a series of static and dynamic contact angle measurements.
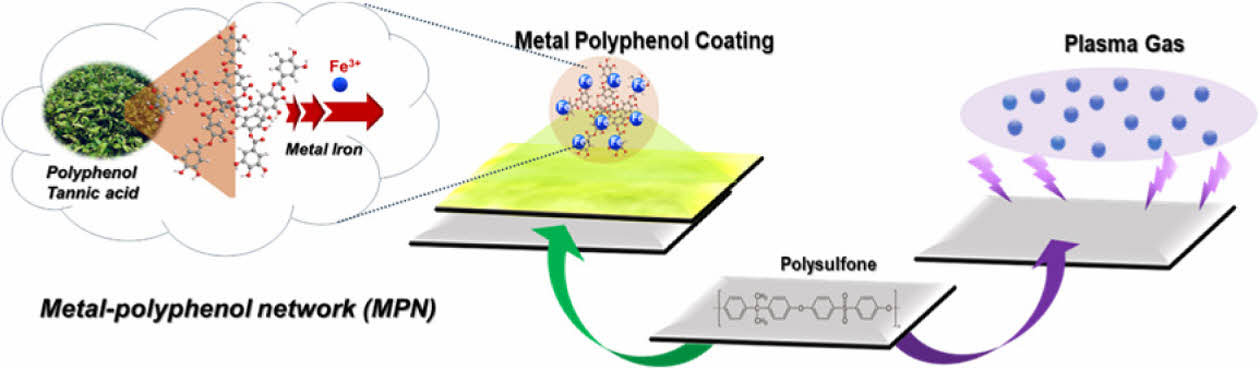
|
Fig. 1 Graphical illustration of surface modification by MPN surface coating and plasma-treatment |
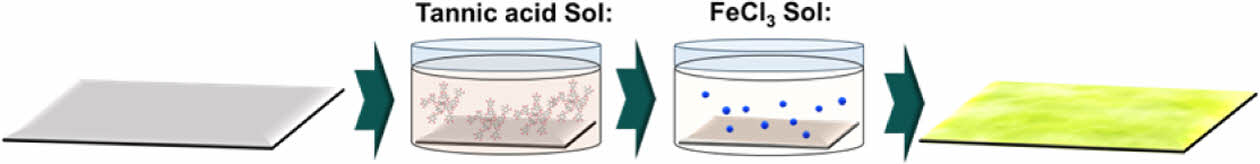
|
Fig. 2 Illustration of modification to TFM by MPN surface coating |

|
Fig. 3 Surface modification to TFM by Plasma treatment with CUTE Plasma system |
3.1 Morphology of TFMs
Surface modification techniques including surface coating with active materials and plasma treatment have been widely utilized for membrane surface functionalization to enhance the wettability, adhesion, polarity, membrane fouling, and separation performance [27]. Understanding surface morphology after the modification can help in explaining the enhanced surface performance and chemical properties of the membranes aimed for water treatment. Fig. 4 presents the FE-SEM images of the top surfaces of un-treated, MPN-coated, and plasma-treated polysulfone TFMs prepared in this study.
More specifically, the un-treated polysulfone membrane (Fig. 4a-c) exhibits a flat surface with several physical pores. After coating MPN (i.e., an active layer of metal polyphenol network complexed by tannic acid and ferric chloride) particles on the polysulfone membrane surface with several spherical-like structures representing MPN particles can be found on the membrane surface (Fig. 4d-i). Of course, these MPN-containing active layers were well coated onto the polysulfone membrane which can be seen in the color change among these un-treated (Fig. 4a) and MPN-coated (Fig. 4d and Fig. 4g) membranes. Clearly, the characteristics of the layering are varied with the amount of the MPN coating (Fig. 4d and Fig. 4g). For the thin layering of MPN onto the polysulfone membrane (Fig. 4d-f), the membrane surface is evenly embraced with the MPN spheres while most of the pores of the virgin membranes are still opened and only surrounded by the MPN particles. Meanwhile, for the thick layering of MPN onto the polysulfone membrane (Fig. 4g-i), the dense MPN layer encloses the membrane pores with stacked MPN particles.
In addition, the variations in morphological change after the plasma treatment are also presented here with the two cases of 60 s plasma treatment and 120 s plasma treatment (Fig. 4j-o). In both cases, a significant widening of pores was resulted. The results also suggested that the membrane pores become more enlarged during the longer-time- plasma treatment. The enlargement of the pores strongly governs the hydrophilicity enhancement of the membrane surface and permeability in the filtration performances [4]. As such, the use of coating and plasma treatment are successfully proposed for the polymeric membrane surface, i.e., polysulfone TFM.
3.2 Surface Roughness of TFMs
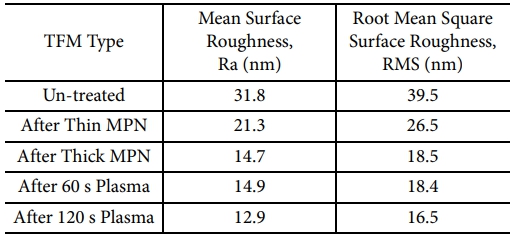
The surface topologies of the un-treated and treated polysulfone TFMs were also observed by the AFM tapping method. The bright areas represent the highest points over the membrane surface while the dark parts refer to the downside valleys. The mean surface roughness and root mean square surface roughness calculated from the AFM images are also presented in Table 2. The un-treated membrane showcases the surface with the frequent nodules produced from the agglomeration of the polymer molecules, which is the typical structure of the membranes fabricated by the phase inversion (Fig. 5a).
After the both post-treatments of MPN coating and plasma treatment, the TFM surface roughness undergoes variations resulting the smoother surfaces (Fig. 5b-e). For the thick layering of MPN onto the TFM surface (Fig. 5c), the surface roughness is significantly reduced, probably arising from the stacked MPN particles fully covering the nodules over the virgin surface. However, the reduction in the surface roughness is not significant for the case of thin MPN-coated polysulfone TFM (Fig. 5b), possibly due to the insufficient thickness of the MPN layer to cover up the polymer aggregates. For the cases of the plasma-treated polysulfone TFMs, the surface roughness was reduced by approximately 53% and 59% corresponding to 60 s and 120 s plasma periods. As such, the surface roughness of the polymeric membranes can be decreased after the plasma treatment emerged from the fact that plasma treatment can etch away the surface irregularities and clean the surface defects. The smoother membrane surface arose by the surface coating or plasma treatment can contribute to the enhancing hydrophilicity and antifouling properties of the membranes for water treatment [28,29]. Clearly, these also agree with those of the above SEM analysis.
3.3 Surface Hydrophilicity and Wetting Behavior of TFMs
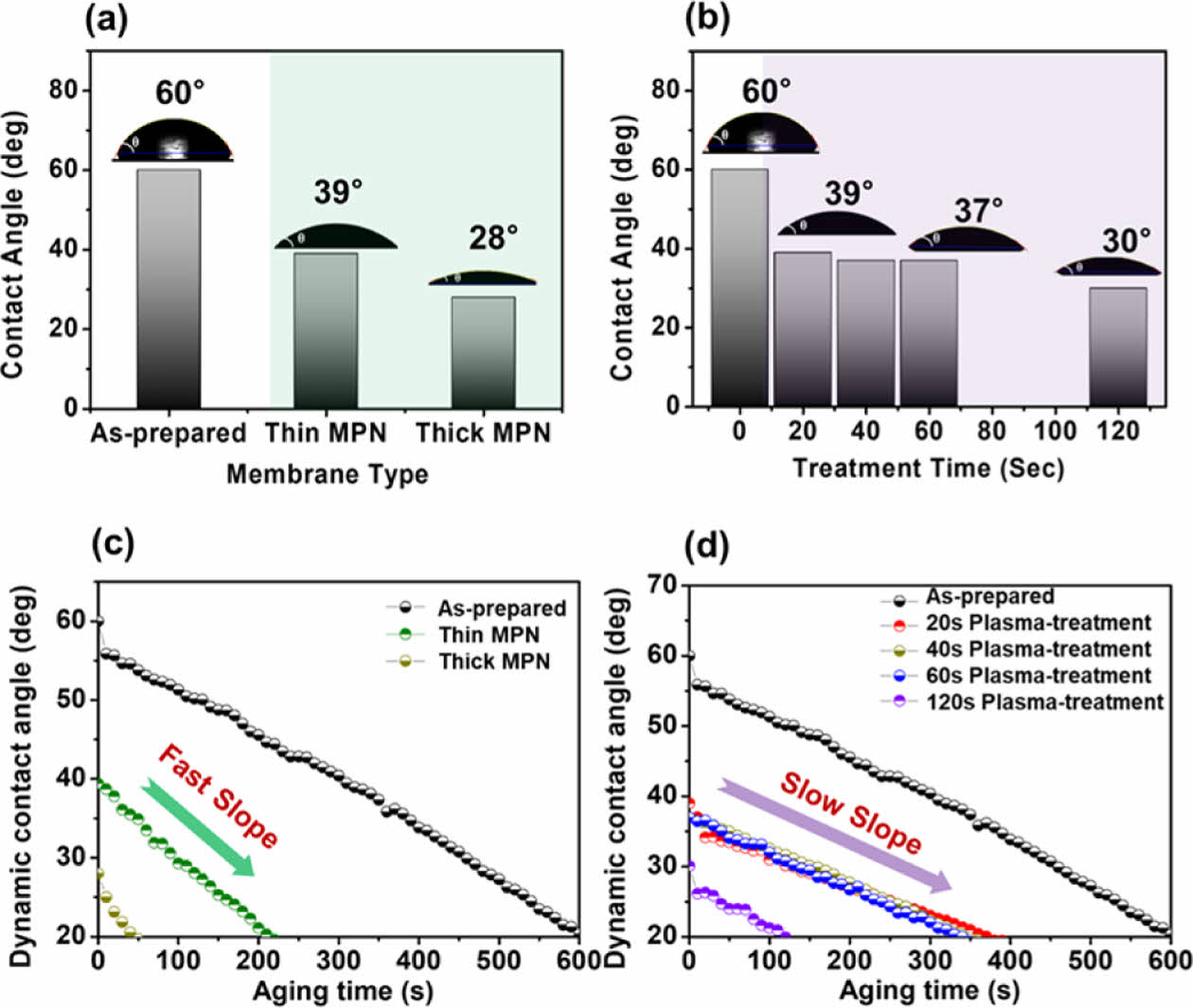
Hydrophilicity plays a critical factor in an antifouling surface by forming a fouling resistance structure on the surface as dominant numbers of fouling resulted from the deposition of hydrophobic pollutants from water. The hydrophilicity of the TFM surfaces was observed by the water contact angle measurements (Fig. 6a,b). The un-treated TFM possessed a water contact angle of 60°. After conducting the MPN coating process on the TFM surface, the water contact angle (WCA) is significantly reduced to 39° and 28° corresponding to the thin and thick MPN-coated TFMs. As known, the metal polyphenol network possesses abundant hydrophilic functional groups such as hydroxyl (-OH) groups and metal-oxygen complexes, which probably indicates the good incorporation of MPN active particles and the TFM surface, as well as an increase in surface hydrophilicity of the polymeric membrane. Similarly, after conducting the surface plasma treatment at 20, 40, 60, and 120 sec, WCA values decreased to 39°, 37°, 37°, and 30°, respectively. As such, the use of a plasma treatment approach on the membrane surface significantly impacted the polymeric membrane during the initial period range of 0-20 sec), meaning that the 20 sec of plasma period was possibly enough to reach the hydrophilic equilibrium of the membrane surface. Besides, the effect of the hydrophilic behavior slightly impacts the polymeric membrane after 20 sec of the plasma period (i.e., 40 sec; 60 sec and 120 sec), in which the WCA of the plasma-treated membranes does not much change from 40 sec to 120 sec period. These indicated an optimal hydrophilicity of the plasma-treated TFMs occurred at 20 sec period instead of longer term of plasma treatment for the polysulfone membrane, meaning that the amount of hydrophilic groups/bonds (–O or –OH groups) is enough and difficult to be further formed onto the surface after longer-term plasma treatment, similarly for the optimal enlarging membrane pores of the 20 s plasma-treated membrane.
Interestingly, the wetting behavior driven by the two post-treatment technologies differs even though both cases resulted in significant hydrophilicity enhancement. Dynamic contact angle gives an insight into the wetting-dewetting membrane over time. Herein, the series of dynamic contact angle measurements were carried out for 600 s to know the adhesion and wetting aspects of the un-treated and treated TFMs surfaces. Wetting of the MPN-coated surface with a water drop exhibits a fast slope (Fig. 6c) whereas the plasma treated surfaces exhibit slow slopes (Fig. 6d). The fast slope is caused by the fast adhesion of water drop representing the quick wetting dynamics and the slow slope is derived from the slow spread of water drop, slow wetting dynamics. As such, whether the thin layering or thick layering of MPN, there are fewer irregularities or hydrophobic obstacles for the liquid movement suggesting lower hysteresis. In contrast, over the plasma-treated surface, the wetting of water drop is slowed down driven by the presence of surface irregularities. Even though the surface roughness is reduced after the plasma treatment, the slow wetting is caused by the stretched pores over the surface or by the phenomenon of hydrophobic recovery over time, where the plasma-treated TFM surface slowly reverting to the original state. Water wetting dynamics is also an important parameter for the membranes designed for water treatment as it directly impacts the membrane’s water permeability and fouling resistance which decreases the membrane life span and rejection and filtration performances [30].
3.4 Bulk Properties of TFMs
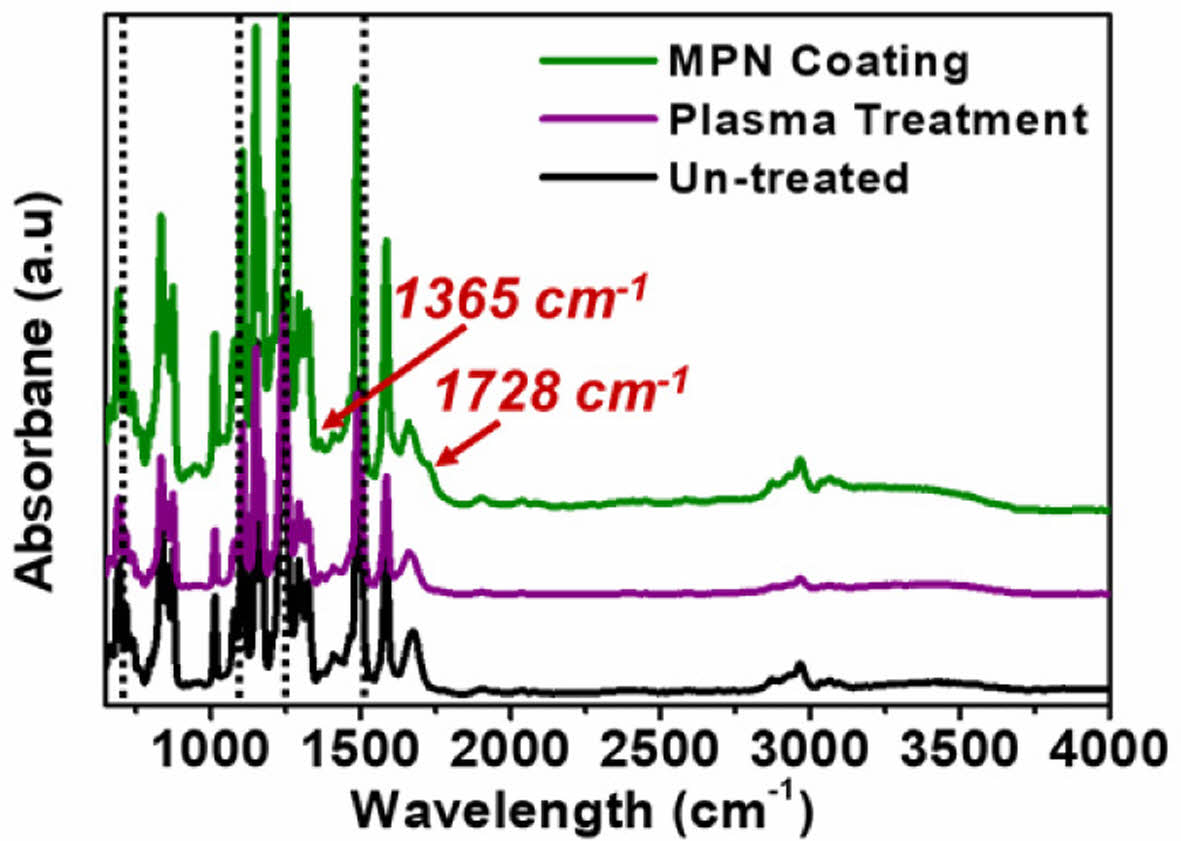
The morphologies, surface roughness, and wetting dynamics of the TFMs are altered by the post-treating with MPN coating and plasma gas. Therefore, the un-treated and surface-modified TFMs were monitored by an ATR FT-IR measurement in order to comprehend whether the two surface modifications alter the bulk properties. As shown in Fig. 7, it gives the information of functional groups present in the target sample giving the chemical characterization of the polysulfone membrane surface before and after the surface treatments (i.e., technical approaches of coating and plasma).
For the un-treated polysulfone membrane, a peak of O-S-O stretching vibration is observed at 1151 cm-1, whereas, peaks of C-O-C and C-C (i.e., aromatic ring) stretching vibrations are corresponding to wavenumbers of 1244 cm-1 and 1585 cm-1, respectively. For the MPN-coated polysulfone membrane, the peaks representing the metal polyphenolic functional groups were observed clearly, for example, main peaks assigned at 1728 cm-1 and 1365 cm-1 regarding to stretching vibrations of C=O band of tannins and C-OH bonding of phenols, respectively. Besides, the main peaks of polysulfone, i.e., O-S-O (1151 cm-1), C-O-C (1244 cm-1) and C-C (1585 cm-1) stretching vibrations, were also detected in the spectrum of MPN-coated membrane. For the plasma-treated polysulfone membrane, the use of 120 sec-plasma treatment was sufficiently enough to reach the hydrophilic equilibrium of the polysulfone membrane surface. However, there was no change in wavenumbers of O-S-O, C-O-C and C-C groups among the un-treated and 120 s plasma-treated polysulfone TFMs. Additionally, the absorbance intensity of these peaks had some differences among the polysulfone membrane surface before and after the surface treatments (i.e., technical approaches of coating and plasma).
Overall, the results showed that even though surface hydrophilicity has enhanced significantly, the bulk properties of the polysulfone TFMs were not distorted by the plasma treatment. And, another factor is that the surface modification depth achieved by the plasma-treatment is lower than the penetration depth by the FT-IR ray. The understanding of the bulk properties of the membranes is also critical as these arise due to the variations in mechanical strength, chemical interaction to the foulants and longevity of the membranes.
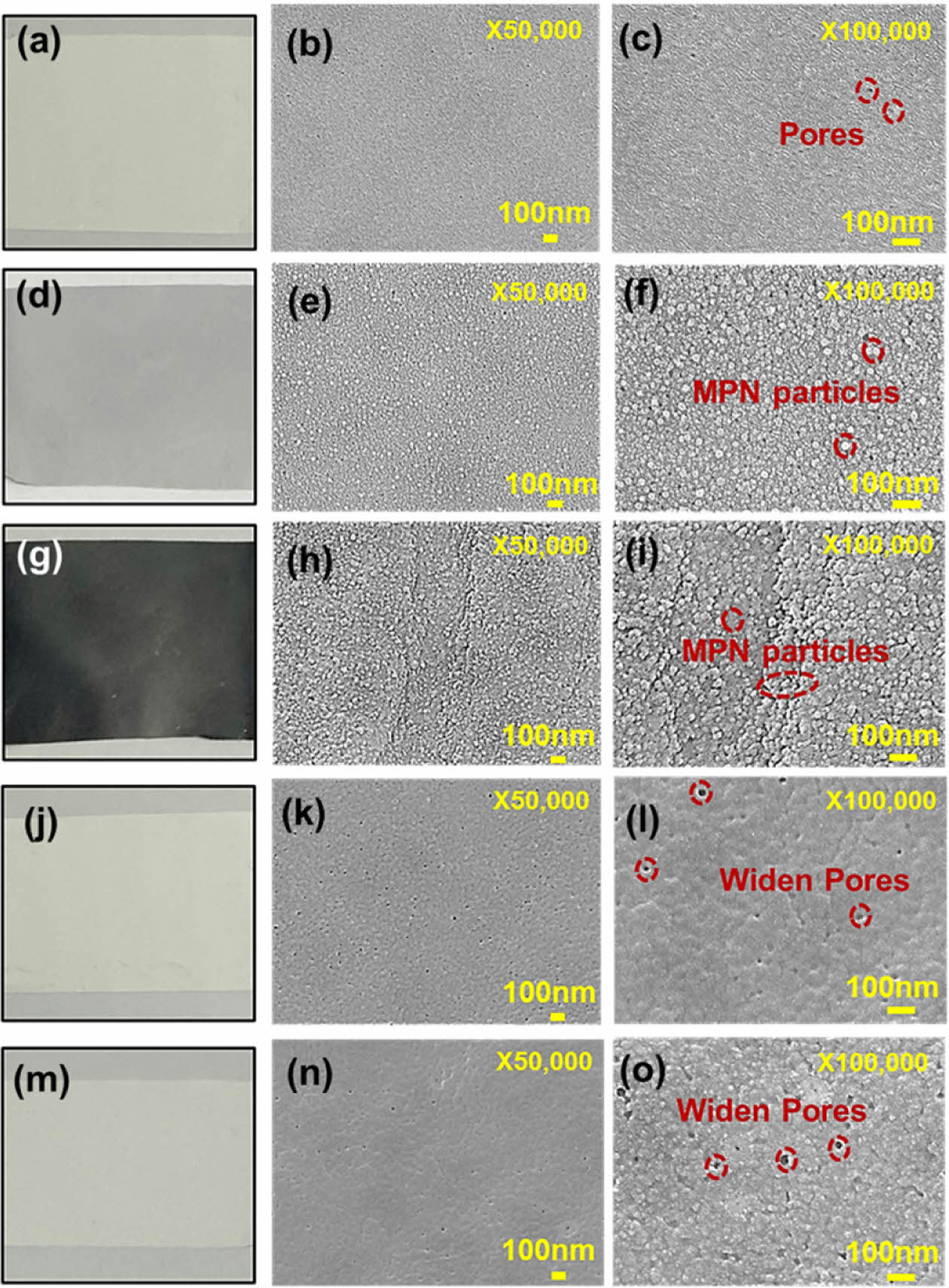
|
Fig. 4 Digital and FE-SEM images of un-treated TFMs (a-c), after thin MPN surface coating (d-f), after thick MPN surface coating (g-i), after 60 s plasma treatment (j-l), and after 120 s plasma treatment (m-o) |
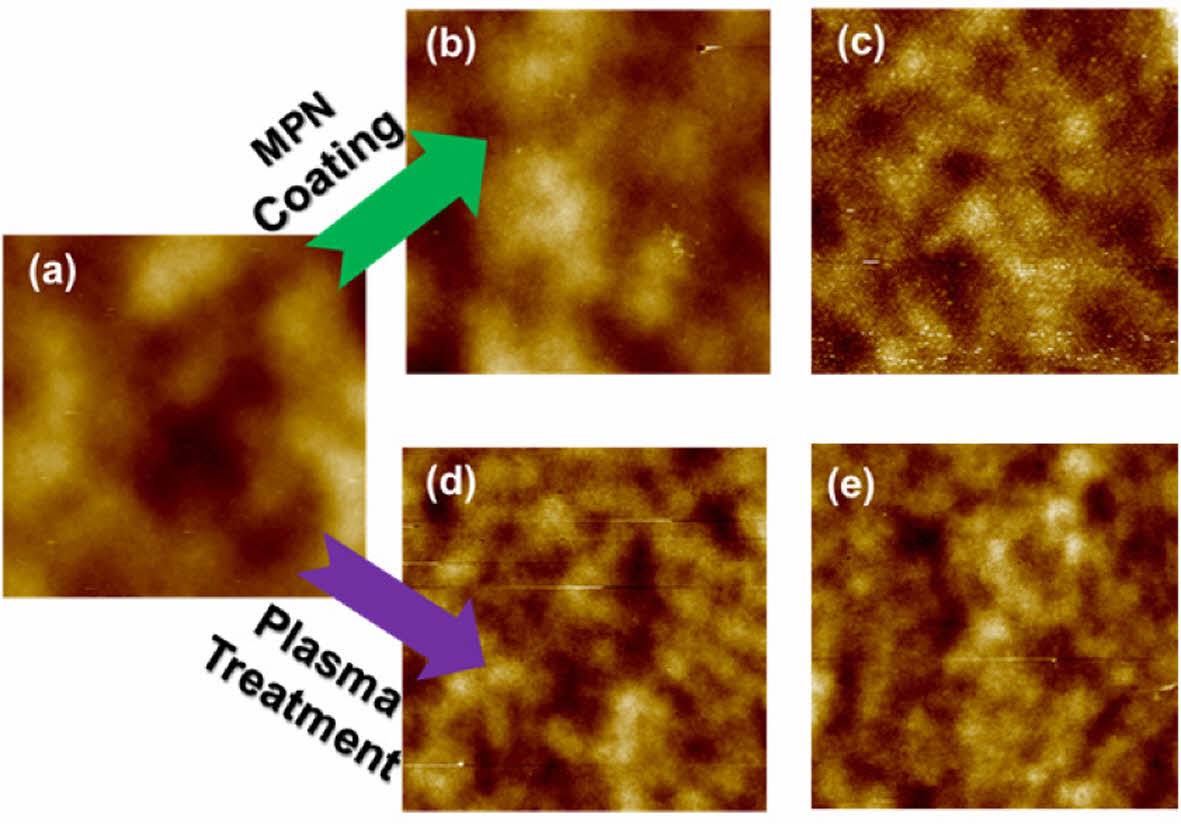
|
Fig. 5 AFM images of un-treated TFMs (a), after thin MPN surface coating (b), after thick MPN surface coating (c), after 60 s plasma treatment (d), and after 120 s plasma treatment (e) (Scanned area 10 μm x 10 μm) |
|
Fig. 6 Hydrophilicity and wetting behavior of TFMs driven by MPN surface coating (a,c) and plasma treatment (b,d) |
|
Fig. 7 FT-IR ATR spectra of TFMs: Un-treated polymer membrane, after plasma treatment and after MPN surface coating |
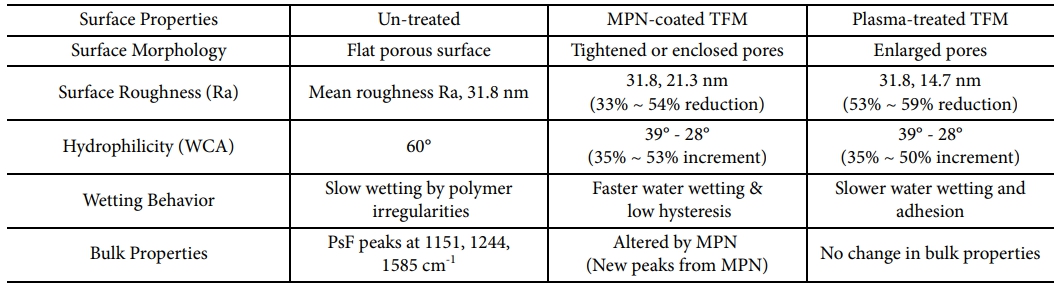
In summary, the comparative study of the two surface modification technologies: surface coating and plasma treatment, for the enhancing hydrophilicity of the TFMs designed for the water treatments was progressed here. For the coating material, the metal polyphenol, MPN, as a self-adherable green coating material was successfully applied. The comprehensive analysis of surface morphology, surface roughness, wetting dynamics, and bulk properties which are the critical parameters for the filtration membranes highlights the significant impacts varied by the two post-treatment methods. The change in the surface properties by two surface modifications technologies are summarized in Table 3. The modifications of the virgin polysome membrane to the functionalized surface demonstrate the variations in the porous structure of the TFMs with surface coating by tightening the membrane pores and plasma treatment by stretching out the pores. Surface hydrophily which is a notable parameter to eliminate membrane fouling was successfully enhanced to TFMs in this work by 35% to 53% after getting applied to two surface modification technologies. Significantly, the surface roughness can be instantly reduced to 53-59% just by the short plasma treatment, whereas the surface coating offers superior wetting dynamics among the two. Moreover, surface coating resulted in notable variations in the functional groups of the membrane surface which can be directly reflected to the fouling resistance, meanwhile, plasma treatment enhanced the hydrophilicity without changing the bulk properties suggesting that mechanical properties or chemical stability can stay intact. These interesting variations triggered by two post-treatment technologies can give insights for optimizing the membrane design for the desired water treatment applications. For our future work, we will conduct further investigations on permeability, foulant, and oil rejections driven by these above-mentioned modified TFMs.
|
Table 3 Change in surface properties by two surface modification technologies: Surface coating and Plasma treatment |
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF2023R1A2C200561712).
- 1. Vo, T.S., Lwin, K.M. and Kim, K., “Recent Developments of Nano-enhanced Composite Membranes Designed for Wastewater Purification—a Review,” Advanced Composites and Hybrid Materials, Vol. 7, No. 4, 2024, pp. 127.
-

- 2. Banerjee, I., Pangule, R.C. and Kane, R.S., “Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent g by Proteins, Bacteria, and Marine Organisms,” Advanced Materials, Vol. 23, No. 6, 2011, pp. 690-718.
-

- 3. Chang, Y., Ko, C.-Y., Shih, Y.-J., Quémener, D., Deratani, A., Wei, T.-C., Wang, D.-M. and Lai, J.-Y., “Surface Grafting Control of lated Poly(vinylidene fluoride) Antifouling Membrane via Surface-initiated Radical Graft Copolymerization,” Journal of Membrane e, Vol. 345, No. 1-2, 2009, pp. 160-169.
-

- 4. Hazarika, T., Kakati, B., Pal, D., Saikia, R., Rawal, A., Mahanta, M.K. and Biswas, S., “Role of Plasma Process Gas on Permeate Flux entation of Cellulose Nitrate Membrane for Mud Water Treatment,” Scientific Reports, Vol. 14, No. 1, 2024, pp. 6585.
-

- 5. Park, S.Y., Chung, J.W. and Kwak, S.Y., “Regenerable Anti-fouling Active PTFE Membrane with Thermo-reversible “peel-and-stick” philic Layer,” Journal of Membrane Science, Vol. 491, No. 2015, pp. 1-9.
-

- 6. Igbinigun, E., Fennell, Y., Malaisamy, R., Jones, K.L. and Morris, V., “Graphene Oxide Functionalized Polyethersulfone Membrane to e Organic Fouling,” Journal of Membrane Science, Vol. 514, No. 2016, pp. 518-526.
-

- 7. Ajmani, G.S., Goodwin, D., Marsh, K., Fairbrother, D.H., Schwab, K.J., Jacangelo, J.G. and Huang, H.O., “Modification of Low re Membranes with Carbon Nanotube Layers for Fouling Control,” Water Research, Vol. 46, No. 17, 2012, pp. 5645-5654.
-

- 8. Yu, H.Y., He, X.C., Liu, L.Q., Gu, J.S. and Wei, X.W., “Surface Modification of Poly(propylene) Microporous Membrane to Im Its Antifouling Characteristics in an SMBR: O2 Plasma Treatment,” Plasma Processes and Polymers, Vol. 5, No. 1, 2008, pp. 84-91.
-

- 9. Afkham, S., Raisi, A. and Aroujalian, A., “Reducing Fouling of Polyethersulfone Microfiltration Membranes by Corona Air Plasma,” Desalination and Water Treatment, Vol. 57, No. 56, 2016, pp. 26976-26992.
-

- 10. Wang, J., Nie, L., Zhang, C. and Wang, B., “Low-pressure Air Plasma-assisted Acrylic Grafted Polyetherimide Ultrafiltration Membranes with Excellent Oil-water Separation and High Anti-fouling Performances,” Materials Today Communications, Vol. 35, No. 2023, pp. 106325.
-

- 11. Abdi, S. and Nasiri, M., “Enhanced Hydrophilicity and Water Flux of Poly (ether sulfone) Membranes in the Presence of Aluminum Fumarate Metal–organic Framework Nanoparticles: Preparation and Characterization,” ACS Applied Materials & Interfaces, Vol. 11, No. 16, 2019, pp. 15060-15070.
-

- 12. Sun, W., Liu, J., Chu, H. and Dong, B., “Pretreatment and Membrane Hydrophilic Modification to Reduce Membrane Fouling,” Membranes, Vol. 3, No. 3, 2013, pp. 226-241.
-

- 13. Yang, L., Han, L., Ren, J., Wei, H. and Jia, L., “Coating Process and Stability of Metal-polyphenol Film,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 484, No. 2015, pp. 197-205.
-

- 14. Yu, H.-Y., Liu, L.-Q., Tang, Z.-Q., Yan, M.-G., Gu, J.-S. and Wei, X.-W., “Surface Modification of Polypropylene Microporous Membrane to Improve Its Antifouling Characteristics in an SMBR: Air Plasma Treatment,” Journal of Membrane Science, Vol. 311, No. 1-2, 2008, pp. 216-224.
-

- 15. Sadeghi, I., Aroujalian, A., Raisi, A., Dabir, B. and Fathizadeh, M., “Surface Modification of Polyethersulfone Ultrafiltration Membranes by Corona Air Plasma for Separation of Oil/water Emulsions,” Journal of Membrane Science, Vol. 430, No. 2013, pp. 24-36.
-

- 16. Zarshenas, K., Raisi, A. and Aroujalian, A., “Surface Modification of Polyamide Composite Membranes by Corona Air Plasma for Gas Separation Applications,” RSC Advances, Vol. 5, No. 25, 2015, pp. 19760-19772.
-

- 17. Kim, K., Lee, K., Cho, K. and Park, C., “Surface Modification of Polysulfone Ultrafiltration Membrane by Oxygen Plasma Treatment,” Journal of Membrane Science, Vol. 199, No. 1-2, 2002, pp. 135-145.
-

- 18. Jaleh, B., Parvin, P., Wanichapichart, P., Saffar, A.P. and Reyhani, A., “Induced Super Hydrophilicity due to Surface Modification of Polypropylene Membrane Treated by O2 Plasma,” Applied Surface Science, Vol. 257, No. 5, 2010, pp. 1655-1659.
-

- 19. Yared, I., Wang, S.-L. and Wang, M.-J., “Effects of Oxygen Plasma and Dopamine Coating on Poly(vinylidene fluoride) Microfiltration Membrane for the Resistance to Protein Fouling,” IEEE Transactions on Plasma Science, Vol. 42, No. 12, 2014, pp. 3847-3857.
-

- 20. Gancarz, I., Poźniak, G. and Bryjak, M., “Modification of Polysulfone Membranes: 3. Effect of Nitrogen Plasma,” European Polymer Journal, Vol. 36, No. 8, 2000, pp. 1563-1569.
-

- 21. Juang, R.-S., Huang, C. and Hsieh, C.-L., “Surface Modification of PVDF Ultrafiltration Membranes by Remote Argon/methane Gas Mixture Plasma for Fouling Reduction,” Journal of the Taiwan Institute of Chemical Engineers, Vol. 45, No. 5, 2014, pp. 2176-2186.
-

- 22. Wavhal, D.S. and Fisher, E.R., “Hydrophilic Modification of Polyethersulfone Membranes by Low Temperature Plasma-induced Graft Polymerization,” Journal of Membrane Science, Vol. 209, No. 1, 2002, pp. 255-269.
-

- 23. Wang, P., Tan, K., Kang, E. and Neoh, K., “Plasma-induced Immobilization of Poly(ethylene glycol) onto Poly(vinylidene fluoride) Microporous Membrane,” Journal of Membrane Science, Vol. 195, No. 1, 2002, pp. 103-114.
-

- 24. Chen, H. and Belfort, G., “Surface Modification of Poly(ether sulfone) Ultrafiltration Membranes by Low‐temperature Plasma‐induced Graft Polymerization,” Journal of Applied Polymer Science, Vol. 72, No. 13, 1999, pp. 1699-1711.
-

- 25. Bryjak, M., Gancarz, I., Poźniak, G. and Tylus, W., “Modification of Polysulfone Membranes 4. Ammonia Plasma Treatment,” European Polymer Journal, Vol. 38, No. 4, 2002, pp. 717-726.
-

- 26. Wavhal, D.S. and Fisher, E.R., “Modification of Polysulfone Ultrafiltration Membranes by CO2 Plasma Treatment,” Desalination, Vol. 172, No. 2, 2005, pp. 189-205.
-

- 27. Ayyavoo, J., Nguyen, T.P.N., Jun, B.-M., Kim, I.-C. and Kwon, Y.-N., “Protection of Polymeric Membranes with Antifouling Surfacing via Surface Modifications,” Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 506, No. 2016, pp. 190-201.
-

- 28. Zhao, H., Wu, L., Zhou, Z., Zhang, L. and Chen, H., “Improving the Antifouling Property of Polysulfone Ultrafiltration Membrane by Incorporation of Isocyanate-treated Graphene Oxide,” Physical Chemistry Chemical Physics, Vol. 15, No. 23, 2013, pp. 9084-9092.
-

- 29. Reis, R., Dumée, L.F., Tardy, B.L., Dagastine, R., Orbell, J.D., Schutz, J.A. and Duke, M.C., “Towards Enhanced Performance Thin-film Composite Membranes via Surface Plasma Modification,” Scientific Reports, Vol. 6, No. 1, 2016, pp. 29206.
-

- 30. Yao, M., Tijing, L.D., Naidu, G., Kim, S.-H., Matsuyama, H., Fane, A.G. and Shon, H.K., “A Review of Membrane Wettability for the Treatment of Saline Water Deploying Membrane Distillation,” Desalination, Vol. 479, No. 2020, pp. 114312.
-

 This Article
This Article
-
2024; 37(6): 492-498
Published on Dec 31, 2024
- 10.7234/composres.2024.37.6.492
- Received on Nov 14, 2024
- Revised on Dec 17, 2024
- Accepted on Dec 23, 2024
 Services
Services
- Abstract
1. introduction
2. experimental
3. results and discussion
4. conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Kyunghoon Kim
-
* School of Mechanical Engineering, Sungkyunkwan University, Suwon 16419, Republic of Korea
- E-mail: kenkim@skku.edu






 Copyright ⓒ The Korean Society for Composite Materials. All rights reserved.
Copyright ⓒ The Korean Society for Composite Materials. All rights reserved.