- Boronic Ester Crosslinked Gels for High Strain Rate Stress Wave Attenuation
Gyeongmin Park*, Dongwon You*, Jimin An*, Sejin Choi**†, Suwon Bae***†, Jaejun Lee*†
* Department of Polymer Science and Engineering, Pusan National University
** Department of Organic Material Science and Engineering, Pusan National University
*** School of Mechanical Engineering, Pusan National UniversityThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
High strain rate stress wave dissipation is essential for protecting personnel and structures from damage caused by shockwaves, which can lead to catastrophic failures in aerospace, defense, and automotive applications. Developing advanced materials capable of efficiently mitigating these dynamic forces is crucial for enhancing safety and durability. This study explores the development and evaluation of boronic ester crosslinked (BCN) gels for high strain rate stress wave dissipation. The BCN gels were synthesized through free radical polymerization and crosslinked via dynamic boronic ester bonds. These gels exhibit enhanced dissipation performance due to their unique ability to undergo reversible bond dissociation. The BCN gels demonstrate superior energy dissipation capabilities compared to conventional materials such as epoxy and polyurea, particularly at higher crosslinking densities. The gels possess self-healing and reprocessing abilities, adding to their versatility for long-term use and damage recovery. The results highlight BCN gels as a promising material for high-performance stress wave mitigation in dynamic environments.
Keywords: Covalent adaptable network, High strain rate stress wave, Shock wave, Organogels, Damping
The dissipation of high strain rate stress waves is critical for protecting personnel structures, and devices in fields such as aerospace [1,2], defense [3], and automotive industries [4]. Polyurea is widely recognized as an effective material for dissipating high strain rate stress waves [5,6], though previous studies have indicated that its performance could benefit from further enhancement [7]. Dynamic covalent bonds have garnered significant attention due to their potential mechanochemical functions, which enable effective pressure dissipation through reconfigurable chemical structures [8]. Recent studies have shown that boronic ester crosslinking in polydimethylsiloxane (PDMS) can enhance the dissipation of high strain rate stress wave pressure via possible exchange reactions [9]. However, this approach has a significant drawback: while the incorporation of boronic ester crosslinks into PDMS improves energy dissipation, it also increases the material's stiffness. In applications requiring shockwave protection, a lower modulus material is often more desirable, as softer materials tend to offer better flexibility and energy absorption [10].
Gel-type materials have emerged as a promising alternative for high strain rate stress wave dissipation [11], owing to their unique viscoelastic properties [12]. Unlike stiff solids, gel-type materials exhibit a combination of solid-like and liquid-like behavior, enabling them to dissipate energy through reversible deformation [13]. This means that upon impact or dynamic loading, these gels can deform to absorb energy and then recover their original shape, making them highly effective at reducing stress wave propagation. Additionally, their high deformability allows them to accommodate large strains without failure [14], which is critical in applications that involve repeated impacts or dynamic forces.
Another key advantage of gel-type materials is their resistance to fracture under dynamic loading [15]. The soft and flexible nature of gels allows them to absorb and distribute impact forces over a larger surface area, reducing localized stress concentrations that could lead to cracking or failure. Furthermore, these materials are often biocompatible and well-suited for interfacing with soft tissues and biological environments [16], making them ideal for personal protective equipment (PPE) and medical devices. Their tunable mechanical properties, such as crosslinking density can be adjusted to optimize the balance between softness and dissipation efficiency [17], enabling tailored solutions for a wide range of applications.
Herein, we propose a gel-type polymer system synthesized via free radical polymerization, crosslinked through boronic ester bonds. The goal of this research is to develop and evaluate gel-type materials with enhanced stress wave damping performance. By optimizing the material properties via the variation of crosslinking density, this study seeks to achieve effective energy dissipation while maintaining the softness necessary for various practical applications.
2.1 Materials
The polymer backbone of the boronic ester crosslinked networks (BCNs) was synthesized using styrene monomer, 2-hydroxyethyl acrylate (HEA), and benzoyl peroxide (BPO) as the thermal initiator. Ethanol served as the solvent, and boric acid was used to crosslink the polymer backbone chains. All materials were sourced from Sigma-Aldrich. For the preparation of specimens for the laser-induced shockwave test, high-purity aluminum (Al) pellets (99.999%, Taewon SCIENTIFIC Co., Ltd.) were used to deposit thin aluminum layers on the outer surfaces of the specimens. Additionally, Na2SiO3 silicate solution was purchased from Daejung Chemical and utilized for the confining layer.
2.2 Laser-induced shockwave test
To assess the performance of shockwave pressure dissipation, a laser-induced shockwave test was performed. The dissipation efficiency was determined by measuring the peak pressure response corresponding to varying input laser fluences.
In this experimental setup, a high-energy, lamp-pumped Q-switched laser (λ = 1064 nm; CNI Lasers LPS 1064-L 700 mJ) was directed onto an Al absorbing layer. A high-energy focusing lens with a focal length of 150 mm was strategically positioned in front of the specimen to concentrate the laser pulse. The presence of a Na2SO3 (sodium silicate) confining layer facilitated the rapid expansion of the Al layer, generating a longitudinal stress wave, or shockwave. This compressive stress wave propagated through the glass substrate and, upon reaching the free surface, reflected back. The reflection induced tensile stress in both the Al reflective layer and the glass substrate, resulting in measurable displacement of the Al surface.
Out-of-plane displacements were quantified using a Michelson interferometer. The interferometric setup involved a diode-pumped continuous laser (λ = 532 nm; Cobolt Samba) directed towards a beam-splitting cube. One of the split beams was focused onto a 200 nm-thick reflective Al film, while the other beam was reflected off a stationary mirror. Upon recombination at the beam splitter, the interference pattern was directed to a photodiode detector (EOT ET-2030). The resulting voltage signals from the interferometer were captured by a photodetector and subsequently digitized using a 2.5 GHz oscilloscope (Tektronix, DPO 7254) operating at a sampling rate of 40 GS/s.
2.3 Preparation of boronic ester crosslinked polymers and gels
A preparation procedure for BCN-X-gel is to mix Styrene, HEA, and Boric acid into a round-bottom flask equipped with a magnetic stirring bar. The mass of each component is shown in Table 1. Subsequently, Ethanol was added to the flask, and the mixture was stirred for 5 minutes. Afterward, BPO (2 wt%) was introduced into the flask and stirred for 12 hours at 80°C. When the reaction is complete, the solvent is evaporated under vacuum to give the polymer (BCN-X). An excess of ethanol was added to the polymer and stirred at 50°C for 12 hours. Excluding the precipitated material, the remaining ethanol was separated to give BCN-X-gels containing 35 wt% ethanol. For the measurements of shockwave pressure dissipation, a 20 mg piece of cured BCN-gels was placed onto a 400 nm thick aluminum deposited glass substrate. A 50 μm polyimide spacer was used to maintain the thickness of the BCN-gel film. A second glass substrate, coated with a 400 nm thick layer, was then placed on top, sandwiching the film between the two glass substrates using with a gentle pressure for an hour at room temperature, 25°C. BCN-gel specimen was prepared without increasing temperature. Water glass Na₂SiO₃ layer was spun cast with 1500 rpm for 45 seconds before conducting the test.
2.4 Chemical and thermal characterization
The synthesized polymers and gels were characterized using Fourier-transform infrared spectroscopy (FT-IR, iS20, Thermo Fisher Scientific Inc), with spectra obtained in the range of 4000 to 750 cm-1 in attenuated total reflectance (ATR) mode to confirm the chemical structure. Differential scanning calorimetry (DSC, DSC 250, TA Instruments) was employed to assess the thermal properties of approximately 5 mg of the BCN polymers. The samples underwent three heating and cooling cycles between -50°C and 110°C at a constant rate of 10°C/min. The glass transition temperatures (Tg) were determined during the second cooling cycle using Trios software.
3.1 Synthesis of BCN polymers and gels
The BCN polymers were successfully synthesized via free radical polymerization, forming a stable crosslinked network. The polymerization involved styrene, HEA and boric acid as the crosslinker, resulting in boronic ester bonds that stabilize the polymer structure. As shown in Fig. 1, these boronic ester crosslinks were crucial in forming the final polymer network. The process was confirmed through both FT-IR spectroscopy and DSC, which verified the successful polymerization and crosslinking.
FT-IR analysis confirms the formation of BCN polymers by highlighting the disappearance of key functional groups in the monomers. As seen in Fig. 2a, the spectrum of BCN-0.50 shows the absence of the C=C stretching vibration (~1632 cm-1) present in the styrene and HEA monomers [18], confirming the completion of the polymerization reaction. Additionally, the reduction of the -OH peak (3400 cm-1~3500 cm-1) suggests successful crosslinking via boronic ester bond formation between boric acid and HEA. These spectral changes provide direct evidence of both polymerization and crosslinking in the BCN polymers.
The thermal properties of the BCN polymers were characterized using DSC, revealing the impact of crosslinking density on glass transition temperature (Tg). Fig. 2b demonstrates that the Tg decreases as the crosslinking density increases: BCN-1.0, BCN-0.67, and BCN-0.50 exhibit Tg values of 48°C, 59°C, and 70°C, respectively. Interestingly, this trend is the opposite of what has been observed in previously reported elastomers, where higher crosslinking density typically results in lower glass transition temperatures. We hypothesize that the rigid styrene component plays a dominant role in determining the Tg, outweighing the effect of crosslinking density. The lower Tg values observed in more highly crosslinked systems, particularly BCN-1.0, suggest increased flexibility and faster segmental motions. This enhanced flexibility makes these polymers well-suited for applications requiring efficient shockwave dissipation.
3.2 Self-healable and reprocessable gels
The BCN gels exhibit remarkable self-healing and reprocessing capabilities, as shown in Fig. 3. After being damaged and split into two pieces, the gels can fully self-heal at room temperature (25°C) without requiring any external stimuli. This self-healing process occurs by simply pressing the damaged parts together, allowing the dynamic boronic ester crosslinks to reform and restore the material's structural integrity. Additionally, once healed, the BCN gels can be reprocessed under mild pressure, enabling them to return to their original shape. These properties make BCN gels highly versatile for applications requiring durability, repairability, and recyclability, as they can repeatedly recover from mechanical damage and be reshaped without losing functionality.
3.3 The generation and measurement of high strain rate stress wave
The generation and measurement of high strain rate stress waves were carried out using a laser-induced shockwave testing setup, as illustrated in Fig. 4 and described in experimental section. The testing apparatus (Fig. 4a) includes a high-energy pulsed laser directed onto a specimen composed of a confining layer, absorbing layer, and a reflective layer. The shockwave generated by the laser pulse is confined within the specimen, inducing high strain rate stress waves that propagate through the material. A Michelson interferometer, coupled with a continuous laser, was used to capture out-of-plane displacements caused by the shockwave, allowing precise measurement of pressure dissipation. The resulting signals were recorded via an oscilloscope and analyzed by Matlab codes.
The calibration setup (Fig. 4b) used a single substrate to measure the input shockwave pressure. A 1 mm thick soda lime glass substrate was employed as a reference material, enabling accurate calibration of the system. The results of the calibration tests (Fig. 4c) show a direct correlation between input laser voltage and the average peak pressure generated during testing. This calibration data validates the accuracy of the pressure measurements and demonstrates the system’s capability to generate controlled shockwaves for further material testing. The average peak pressure obtained from the calibration setup, as shown in Fig. 4c, will serve as the input peak pressure for evaluating the stress wave dissipation performance.
3.4 High strain rate stress wave dissipation performance of BCN gels
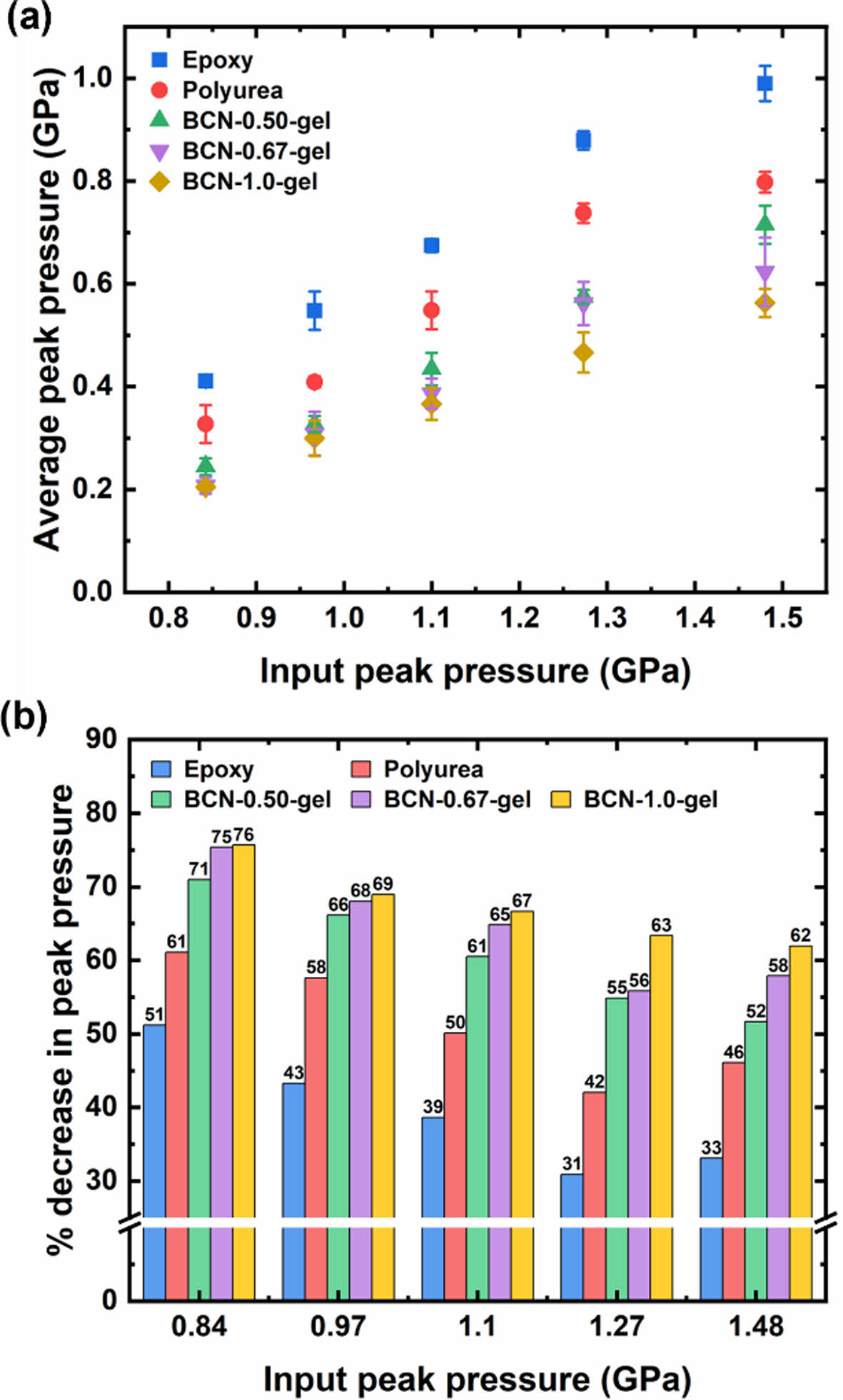
BCN gels exhibit superior dissipation performance compared to epoxy as a composite matrix and polyurea as a benchmark material for the dissipation, particularly at higher crosslinking densities. Fig. 5a shows the average peak pressure as a function of input peak pressure for the various materials. The results indicate that BCN gels with higher crosslinking density, such as BCN-1.0-gel, consistently display lower average peak pressures. This suggests that the enhanced crosslinking in these gels improves their capacity to dissipate stress wave energy, leading to a significant reduction in the pressure transmitted through the material. In contrast, epoxy and polyurea exhibit higher average peak pressures, reflecting their lower efficiency in energy dissipation.
The percentage decrease in peak pressure further highlights the superior dissipation capabilities of BCN gels. As depicted in Fig. 5b, the BCN-1.0-gel, followed by BCN-0.67-gel and BCN-0.50-gel, shows a greater percentage decrease in peak pressure compared to epoxy and polyurea. This trend confirms that higher crosslinking density results in better shockwave energy dissipation, which supports the hypothesis that exchange or dissociation reactions of boronic ester bonds contribute to the pressure dissipation. The exceptional performance of the BCN-1.0-gel, with the highest percentage reduction in peak pressure, underscores its potential for use in applications requiring robust stress wave mitigation as a gel state.
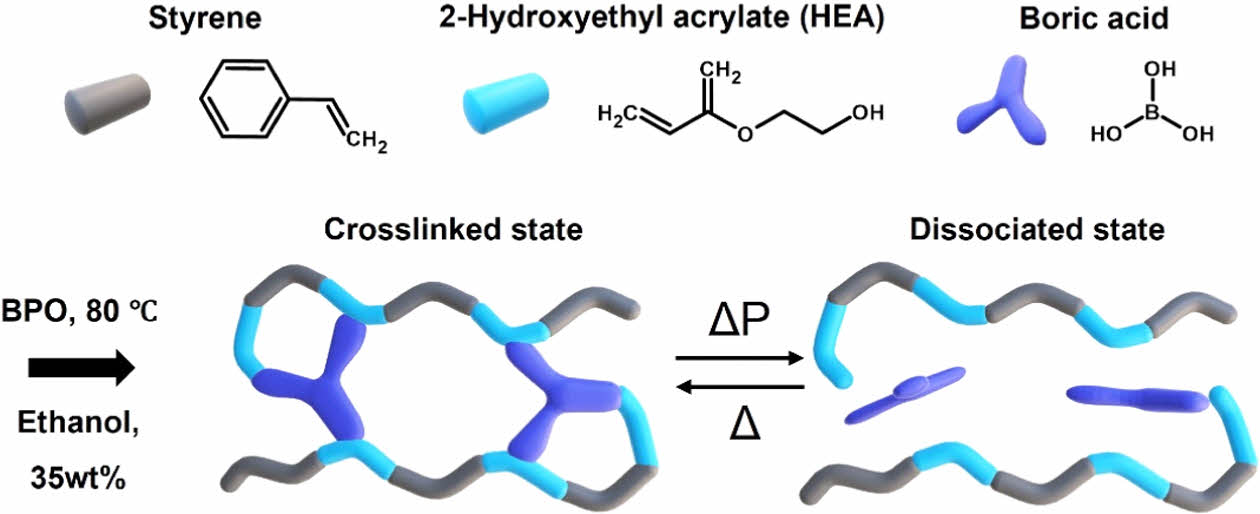
|
Fig. 1 Synthesis of boronic ester crosslinked network (BCN) and its proposed stress dissipation mechanism. The BCN is synthesized via the polymerization of styrene, 2- hydroxyethyl acrylate (HEA), and boric acid in ethanol (35 wt%) as the solvent, with benzoyl peroxide (BPO) as the thermal initiator at 80°C. The resulting material transitions between a crosslinked state and a dissociated state under applied pressure or temperature. The boric acid facilitates dynamic crosslinking, enabling the network to dissipate stress through reversible bond dissociation under shock loading (ΔP) |
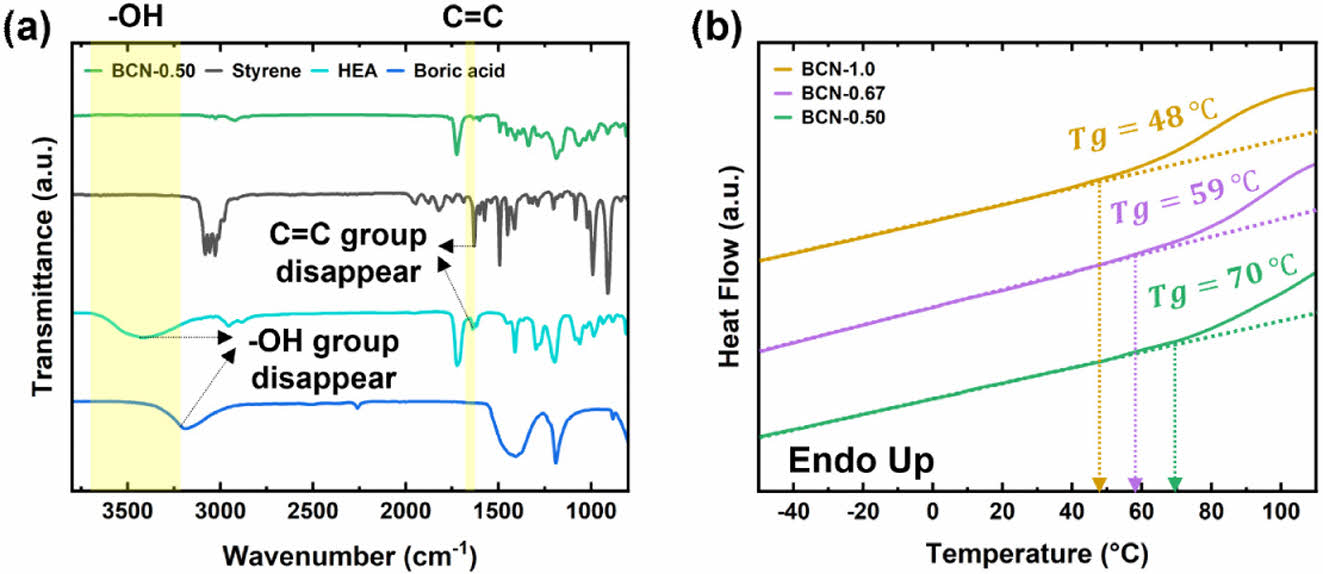
|
Fig. 2 Chemical and thermal properties of BCN polymers. (a) FTIR spectra illustrating the polymerization of BCN materials. The disappearance of the C=C bond peak at 1632 cm-1 confirms the successful polymerization of styrene and HEA. Additionally, the disappearance of the O–H group peak at 3400 cm-1~3500 cm-1 indicates the formation of boronic ester linkages through crosslinking between boric acid and hydroxyl groups in HEA. (b) Differential scanning calorimetry (DSC) analysis showing the glass transition temperatures (Tg) of BCN-1.0, BCN-0.67, and BCN-0.50, measured at 48°C, 59°C, and 70°C, respectively. The decreasing Tg values correspond to higher degrees of crosslinking density in the BCN formulations |
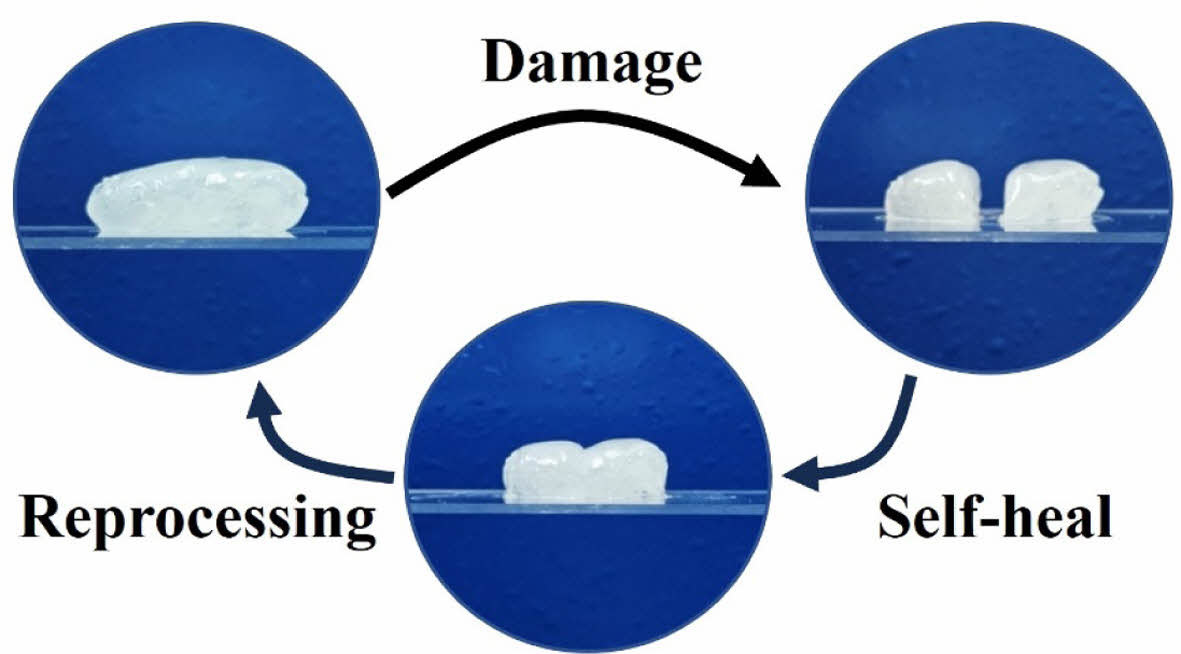
|
Fig. 3 Self-healing and reprocessing capabilities of BCN-1.0-gels. The BCN gels demonstrates excellent self-healing properties. After being damaged and split into two pieces, the material can self-heal by simply pressing the pieces together at room temperature (25°C) without the need for external stimuli. Once healed, the gels can undergo reprocessing under mild pressure, reshaping it into its original form |
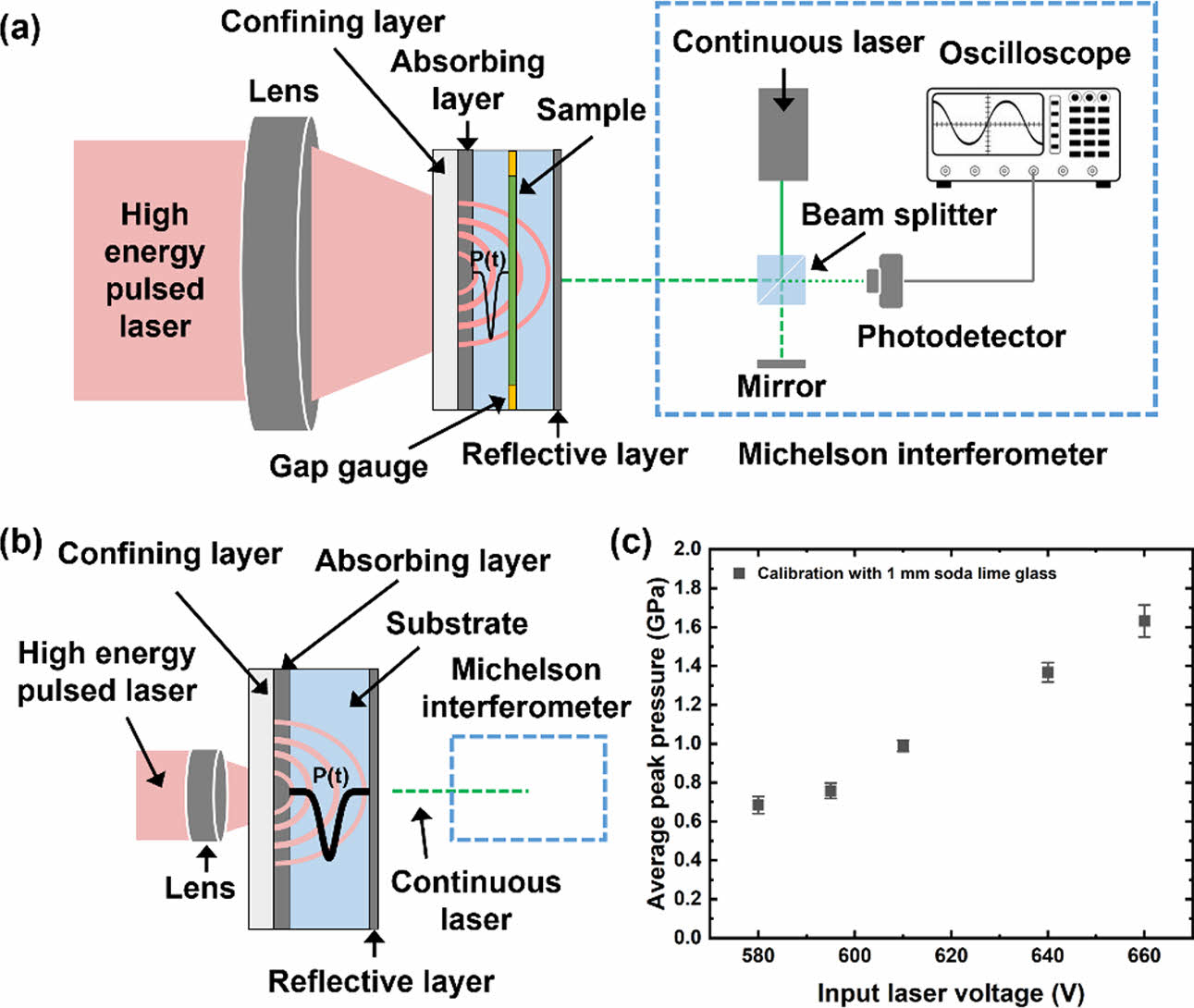
|
Fig. 4 Schematics of the laser-induced shockwave test and calibration setup. (a) The apparatus for laser-induced shockwave testing includes a high-energy pulsed laser, a confining layer, absorbing layer, and a sandwich-structured specimen with a reflective layer for measuring pressure dissipation. The Michelson interferometer is used to capture shockwave signals with a continuous laser setup, interfacing with an oscilloscope. (b) Calibration test setup with a single substrate specimen to measure input shockwave pressure. (c) Calibration results showing average peak pressure as a function of input laser voltage, using a 1 mm soda lime glass substrate for reference. The data illustrate the relationship between input laser voltage and average peak pressure in the calibration tests |
|
Fig. 5 High strain rate stress wave dissipation performance of BCN gels. (a) Average peak pressure as a function of input peak pressure for epoxy, polyurea, and BCN gels. BCN gels with higher crosslinking density (BCN-1.0-gel) demonstrate lower average peak pressures, corresponding to enhanced pressure dissipation capability. (b) Percentage decrease in peak pressure relative to input peak pressure for the different materials. BCN gels, particularly those with higher crosslinking density, show superior performance, with a greater percentage decrease in peak pressure compared to epoxy and polyurea |
In conclusion, boronic ester crosslinked (BCN) gels have demonstrated exceptional high strain rate stress wave dissipation performance, surpassing traditional materials like epoxy and polyurea. The dynamic nature of boronic ester bonds likely enables effective energy dissipation through reversible bond dissociation, especially at higher crosslinking densities. This research also confirmed that increasing crosslinking density enhances the gels' ability to mitigate the pressure while maintaining their flexibility and softness. Additionally, the self-healing and reprocessing capabilities of the BCN gels further emphasize their potential for long-term use in environments where mechanical damage is likely. These findings suggest that BCN gels are a highly promising material for applications in aerospace, defense, automotive, and other fields requiring robust shockwave protection. Future research could explore further optimization of the gel formulations and their performance under different dynamic loading conditions.
This work was supported by a 2-Year Research Grant of Pusan National University.
- 1. Peters, A.B., Zhang, D., Chen, S., Ott, C., Oses, C., Curtarolo, S., McCue, I., Pollock, T.M., and Eswarappa Prameela, S., “Materials n for Hypersonics,” Nature Communications, Vol. 15, No. 1, 2024, pp. 3328.
-

- 2. Eswarappa Prameela, S., Pollock, T.M., Raabe, D., Meyers, M.A., Aitkaliyeva, A., Chintersingh, K.-L., Cordero, Z.C., and GraBrady, L., “Materials for Extreme Environments,” Nature Reviews Materials, Vol. 8, No. 2, 2023, pp. 81-88.
-

- 3. Weppner, J., Linsenmeyer, M., and Ide, W., “Military Blast-Related Traumatic Brain Injury,” Current Physical Medicine and Rehaion Reports, Vol. 7, No. 4, 2019, pp. 323-332.
-

- 4. Ekström, J., Rempling, R., and Plos, M., “Spalling in Concrete Subjected to Shock Wave Blast,” Engineering Structures, Vol. 122, pp. 72-82.
-

- 5. Yao, K., Liu, Z., Li, T., Guo, B., and Zhuang, Z., “Mesoscale Structure-based Investigation of Polyurea Dynamic Modulus and -wave Dissipation,” Polymer, Vol. 202, 2020, pp. 122741.
-

- 6. Gyeongmin, P., Seungrae, C., Hyejin, K., and Jaejun, L., “Design of Polymer Composites for Effective Shockwave Attenuation”, osites Research, Vol. 37, No. 1, 2024, pp. 21-31.
-

- 7. Ding, L., Wang, Y., Lin, J., Ma, M., Hu, J., Qiu, X., Wu, C., and Feng, C., “Recent Advances in Polyurea Elastomers and Their Apons in Blast Protection: A Review,” Journal of Materials Science, Vol. 59, No. 32, 2024, pp. 14893-14923.
-

- 8. Lee, J., Park, G., Lee, D., Shin, J., Ahn, C.-H., Lee, J., and Kim, T.A., “Principles for Designing Sustainable and High-strain Rate Wave Dissipating Materials,” Materials Horizons, Vol. 11, 2024, pp. 5220-5229.
-

- 9. Lee, J., Jing, B.B., Porath, L.E., Sottos, N.R., and Evans, C.M., “Shock Wave Energy Dissipation in Catalyst-Free Poly(dimethylsiloxane) Vitrimers,” Macromolecules, Vol. 53, No. 12, 2020, pp. 4741-4747.
-

- 10. Vega, D.A., Lance, P., Zorzi, E., Register, R.A., and Gómez, L.R., “Shock Compression of Semiflexible Polymers,” Soft Matter, Vol. 19, No. 32, 2023, pp. 6131-6139.
-

- 11. Veysset, D., Sun, Y., Lem, J., Kooi, S.E., Maznev, A.A., Cole, S.T., Mrozek, R.A., Lenhart, J.L., and Nelson, K.A., “High-Strain-Rate Behavior of a Viscoelastic Gel Under High-Velocity Microparticle Impact,” Experimental Mechanics, Vol. 60, No. 9, 2020, pp. 1179-1186.
-

- 12. Song, Y., Kim, M.G., Yi, H.G., and Lee, D., “Nonlinear Rheological Properties of Endothelial Cell Laden-cellulose Nanofibrils Hydrogels,” Composites Research, Vol. 35, No. 3, 2022, pp. 153-160.
-

- 13. Accardo, J.V., and Kalow, J.A., “Reversibly Tuning Hydrogel Stiffness Through Photocontrolled Dynamic Covalent Crosslinks,” Chemical Science, Vol. 9, No. 27, 2018, pp. 5987-5993.
-

- 14. Huang, J., Xu, Y., Qi, S., Zhou, J., Shi, W., Zhao, T., and Liu, M., “Ultrahigh Energy-dissipation Elastomers by Precisely Tailoring the Relaxation of Confined Polymer Fluids,” Nature Communications, Vol. 12, No. 1, 2021, pp. 3610.
-

- 15. Bao, B., Zeng, Q., Li, K., Wen, J., Zhang, Y., Zheng, Y., Zhou, R., Shi, C., Chen, T., Xiao, C., Chen, B., Wang, T., Yu, K., Sun, Y., Lin, Q., He, Y., Tu, S., and Zhu, L., “Rapid Fabrication of Physically Robust Hydrogels,” Nature Materials, Vol. 22, No. 10, 2023, pp. 1253-1260.
-

- 16. Liu, J., Lin, S., Liu, X., Qin, Z., Yang, Y., Zang, J., and Zhao, X., “Fatigue-resistant Adhesion of Hydrogels,” Nature Communications, Vol. 11, No. 1, 2020, pp. 1071.
-

- 17. Xu, Z., Lu, J., Lu, D., Li, Y., Lei, H., Chen, B., Li, W., Xue, B., Cao, Y., and Wang, W., “Rapidly Damping Hydrogels Engineered Through Molecular Friction,” Nature Communications, Vol. 15, No. 1, 2024, pp. 4895.
-

- 18. Zych, A., Tellers, J., Bertolacci, L., Ceseracciu, L., Marini, L., Mancini, G., and Athanassiou, A., “Biobased, Biodegradable, Self-Healing Boronic Ester Vitrimers from Epoxidized Soybean Oil Acrylate,” ACS Applied Polymer Materials, Vol. 3, No. 2, 2021, pp. 1135-1144.
-

 This Article
This Article
-
2024; 37(6): 441-446
Published on Dec 31, 2024
- 10.7234/composres.2024.37.6.441
- Received on Oct 25, 2024
- Revised on Nov 7, 2024
- Accepted on Nov 14, 2024
 Services
Services
- Abstract
1. introduction
2. experimental section
3. results and discussion
4. conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Sejin Choi**, Suwon Bae***, Jaejun Lee*
-
* Department of Polymer Science and Engineering, Pusan National University
** Department of Organic Material Science and Engineering, Pusan National University
*** School of Mechanical Engineering, Pusan National University - E-mail: sejin@pusan.ac.kr, suwon.bae@pusan.ac.kr, jlee-pse






 Copyright ⓒ The Korean Society for Composite Materials. All rights reserved.
Copyright ⓒ The Korean Society for Composite Materials. All rights reserved.