- Effect of Grafted Biobased Acrylics on the Mechanical Properties of Polylactic Acid (PLA)/Starch Eco-Friendly Composite
Marcela Godoy*, Jonghwan Suhr**†
* Polymer Science and Engineering, Sungkyunkwan University
** Department of Mechanical EngineeringThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Using non-biodegradable polymers is a severe environmental problem as they are not recyclable and generate a large amount of waste. Biopolymers, such as starch-based composites, have been considered one of the most promising replacement materials. These eco-friendly materials have the advantage of being low-cost, biodegradable, and obtained from renewable sources. However, as starch tends to be brittle and hydrophilic, it can make these materials unusable when exposed to water and limit its processability for further applications. In this work, a biobased modified starch was grafted using two bioderived materials, lauryl methacrylate (LMA) and tetrahydrofurfuryl methacrylate (THFMA), by radical polymerization. A polylactic acid (PLA) composite based on the modified starch (m-St) was fabricated to enhance its toughness. These samples were characterized by Fourier transform infrared, 1H NMR and 13C NMR analysis, optical and scanning electron microscopy. The starch was successfully grafted, thus improving the compatibility with the PLA matrix. The mechanical properties of these films were also studied. Results from mechanical tests showed a slight enhancement of the mechanical performance of these composites when m-St was added to the PLA matrix. Such behavior is related to the improved dispersion of m-St 1:2 on PLA, confirmed by SEM images showing enhanced compatibility between modified starch and PLA matrix. This indicated excellent properties of the produced composite film for further eco-friendly applications.
Keywords: Starch, Graft polymerization, Polylactic acid (PLA), Mechanical properties
Research toward using biopolymers from renewable sources has prompted the crucial need to replace petroleum-based plastics with a more sustainable economy due to the current global environmental challenges [1,2]. PLA is a linear aliphatic polyester obtained from renewable sources such as corn and sugar cane, making it an appropriate biological polymer. Polylactic acid (PLA) has good biocompatibility and biodegradability. It eventually decomposes into CO2 and H2O in the environment, causing no environmental challenge [3,4]. To produce biodegradable, low-cost, renewable biocomposites, PLA is often mixed with natural materials.
Starch is a carbohydrate belonging to the polysaccharides family and is one of the most promising biopolymers due to its low cost, biodegradability, and abundance, as it can be extracted from sources unsuitable for human consumption [5]. Nevertheless, native starch (St) shows certain disadvantages, such as high-water absorption and poor mechanical performance, thus difficulty controlling processing.
One of the essential techniques for modifying the physical and chemical properties of St is chemical modification, and an efficient way to modify the physicochemical properties of starch is by graft copolymerization. This alternative method improves several properties of starch by structural alternation and producing new functional groups [6].
Shi et al. mentioned that the compressed thermoplastic starch grafted by hexamethyl acrylate (HMA) exhibited the improvement of flexibility caused by the steric hindrance from a monomer with long side chains [7].
Grafted starch is prepared by graft copolymerization with various monomers such as styrene (S), acrylonitrile (AN), acrylamide (AM), acrylic acid (AA), methyl methacrylate (MMA), and methacrylic acid (MAA) [8,9]. All these monomers affected a variety of functional groups, which altered physicochemical properties and modified structures.
To the extent of current knowledge, there are no reports on grafted starch using these two monomers, lauryl methacrylate (LMA) and tetrahydrofurfuryl methacrylate (THFMA), that besides their biobased characteristics, they can alter starch physicochemical properties attributed to their carbon long chain and functional groups [10].
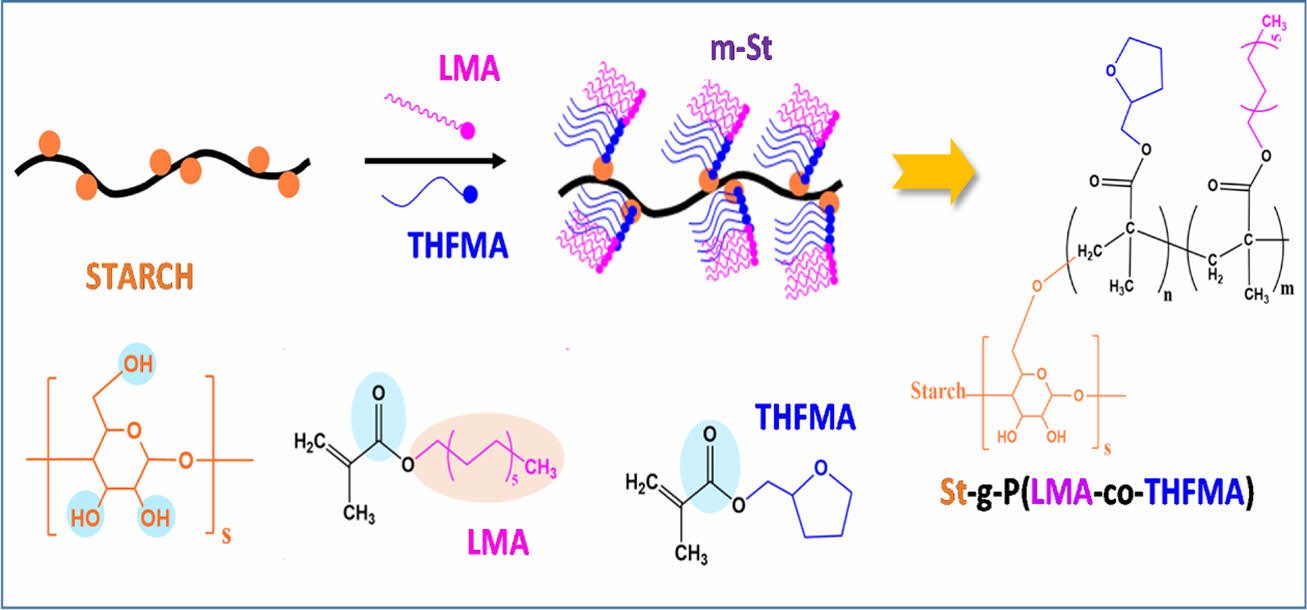
In this study, we reported starch copolymerization with biobased acrylate monomers, lauryl methacrylate (LMA), and tetrahydrofurfuryl methacrylate (THFMA) via chemical graft polymerization to improve processability and compatibility between hydrophilic starch and hydrophobic polymers (Fig. 1). Table 1
|
Fig. 1 Synthesis of m-St or St-g-P(LMA-co-THFMA) |
2.1 Materials
Starch, Lauryl Methacrylate (LMA), and Tetrahydrofurfuryl Methacrylate (THFMA) were purchased from Samchun Pure Chemical Co., Ltd., South Korea. Luminy LX530 PLA was purchased from Total Energies Corbion. Dimethyl sulfoxide (DMSO), hydrogen peroxide (H2O2), calcium chloride (CaCl2), and hydrochloric acid (HCl) were purchased from Samchun Pure Chemical Co., Ltd., South Korea. Starch was dried in a vacuum drying oven at 45°C for 48 h to eliminate moisture before use. All other chemicals were used without any additional purification.
2.2 Synthesis of Starch Grafted Acrylate (m-St)
3.0 g of CaCl2 was added into a dry flask containing 60 mL of dimethyl sulfoxide until completely dissolved. Then, 3.0 g of starch was added to the mixture and stirred for 30 min. Lauryl Methacrylate (LMA) and Tetrahydrofurfuryl Methacrylate (THFMA) were dropped into the solution, and 3.0 mL of H2O2 was added. All the solution was heated to 45°C. After 24 hr., the mixture was slowly poured into a diluted solution of HCl. The precipitate was filtered, washed with methanol, and dried in a vacuum oven at 50°C [11]. Different monomer loading weight ratio was used, thereby obtaining starch with varying amounts of grafted monomer.
The amount of the grafted polymer was calculated as the weight of the sample increases (W2) after extraction of the homopolymer.
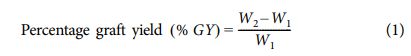
where W1=initial weight of the sample and W2=final weight of the sample after extraction of the homopolymer [12].
2.3 Preparation of PLA/m-St Composite Films
The PLA/St-g-(LMA-co-THFMA) composites were fabricated using a loading ratio of 10 wt % m-St by melt blending using a Thermo Haake mixer at 180°C for 12 min and then hot pressed under 20 MPa for 12 min at the same temperature.
2.4 Characterization and Testing
Fourier transform infrared (FTIR) spectra of the samples were recorded on an ALPHA II - Platinum FT-IR Spectrometer in a range of wavenumbers from 4000 to 800 cm-1 for a total scan time of 64. The 1H NMR and 13C NMR analysis was performed on a Bruker AVANCE 400 NMR spectrometer using DMSO as the solvent.
The contact angle measurement was carried out on a DSA-25 KRUSS instrument at three different points on the sample surface in a controlled ambient room. The surface tensions of starch (γSt) and modified starch (γm-St) were determined to analyze the interfacial tension with PLA. The surface tension γSt and γm-St were calculated by measuring the contact angle of two selected liquids on their surfaces. Theoretically, the disperse (γd) and polar (γp) components of surface tension can be determined from the contact angle using the Owens-Wendt method.
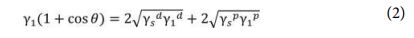
where θ is the contact angle, ¥ã is the surface tension, subscripts ‘s’ and ‘l’ refer to solid samples and liquid, respectively, and superscripts ‘d’ and ‘p’ mean disperse and polar parts, respectively. Distilled water and diiodomethane, are the two used test liquids for determining contact angle [11].
The interfacial tension between starch and PLA can be estimated from their surface tension values by this equation
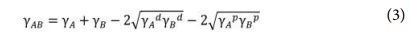
Optical microscope images were collected on an ECLIPSE LV100D Industrial Microscope.
The mechanical properties were measured using a Universal Testing machine INSTRON (E3000) according to the ASTM D882-12 standard with a test rate of 5 mm min-1 and five specimens were tested for each sample. The tensile properties were determined by stress-strain curves [13].
3.1 Synthesis and Characterization of Starch Grafted Acrylate
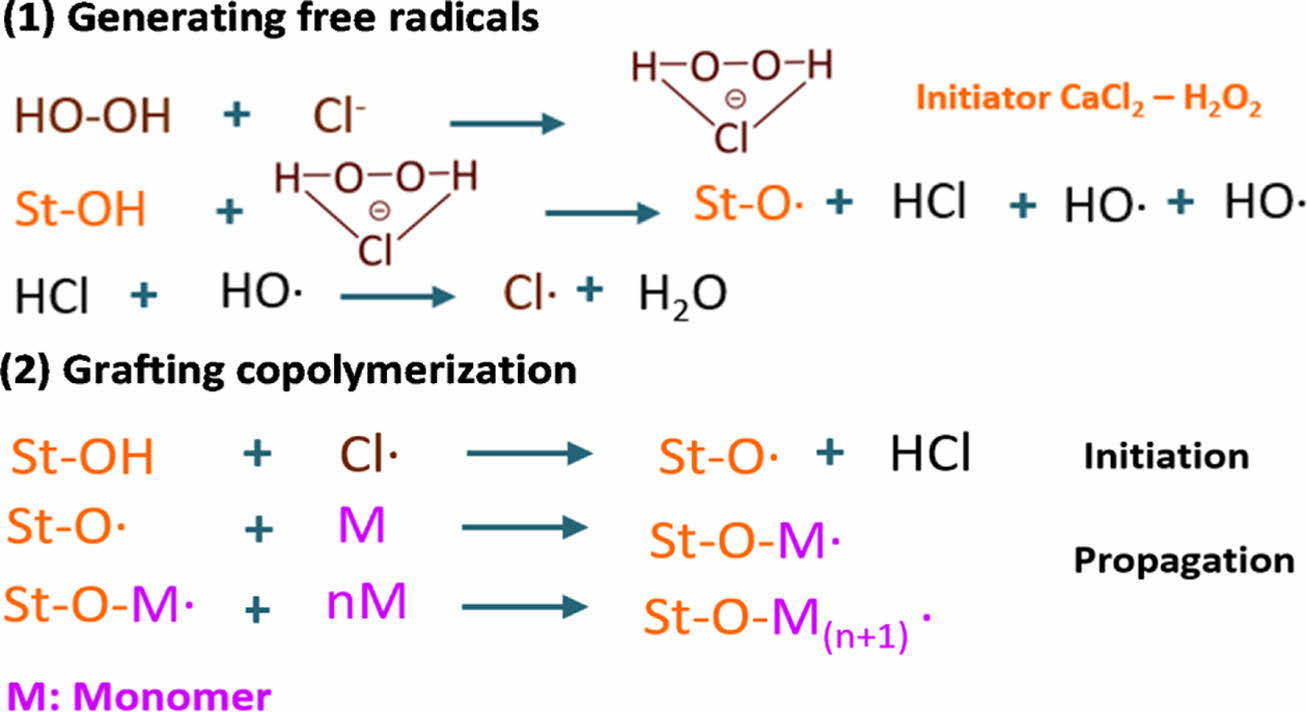
The possible mechanism of the synthesis reaction has been proposed in previous work with some modifications (Fig. 2). In general, the initiators H2O2 and CaCl2 form a hydroperoxide-chloride ion complex, in which the chlorine can abstract the hydrogen from the abundant hydroxide groups in starch to form starch macromolecular radicals, initiating the polymerization. The results validate that this initiator system is efficient for starch grafting polymerization [14].
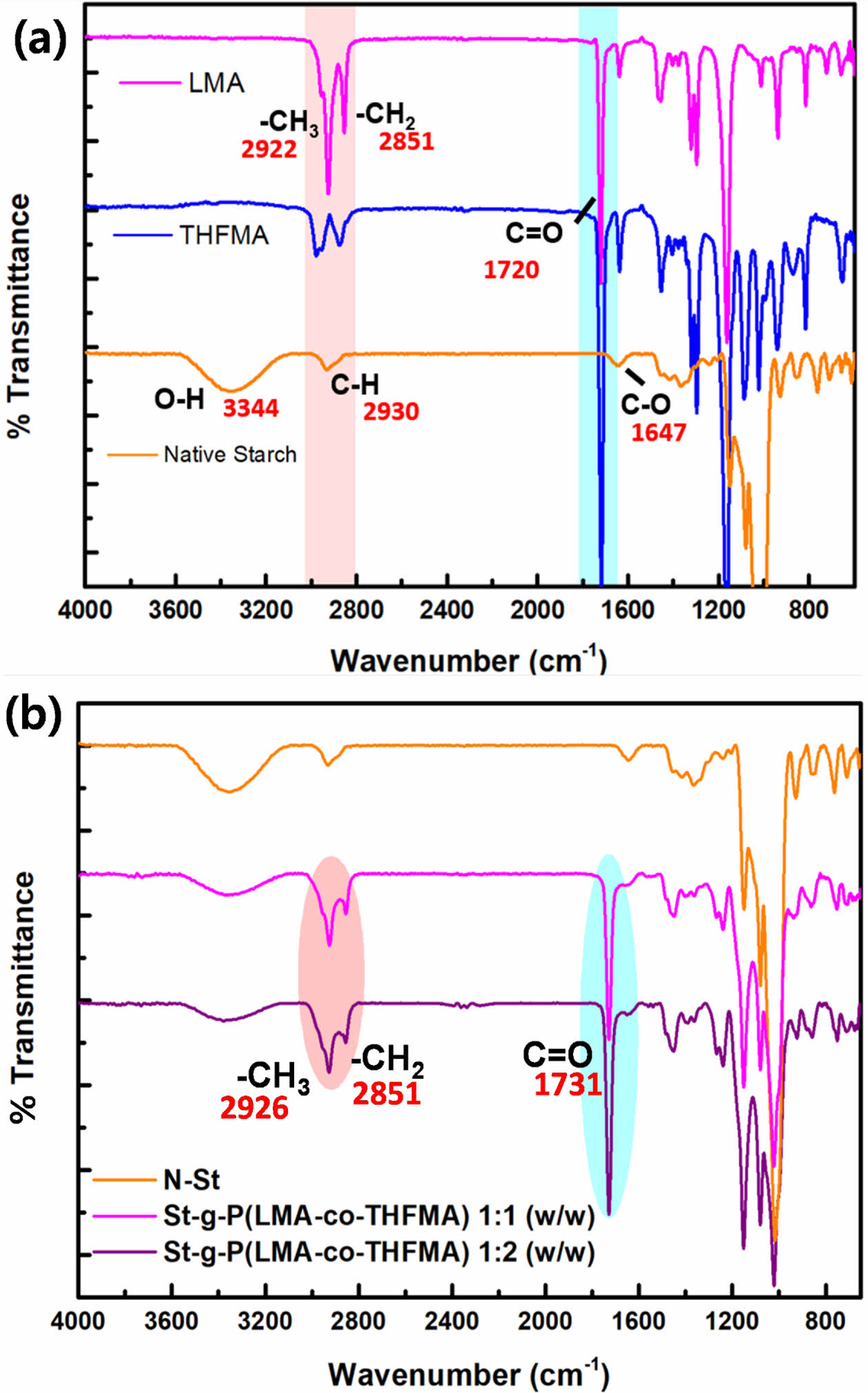
FTIR analysis of native starch and the monomers grafted onto starch was performed to verify that the graft reaction occurred and to investigate the resulting structural changes before and after starch modification. The graft copolymers of starch with LMA and THFMA, St-g-P (LMA-co-THFMA), are shown in Fig. 3.
As shown in Fig. 3(a), FTIR spectra of the native starch indicated the characteristic absorption peaks of the starch backbone; the hydroxyl group OH- around 3344 cm-1 stretching and vibration of the hydrogen bond, 2930 cm-1 corresponding to the C-H asymmetrical stretching and vibration, CO-1647 cm-1 and CH/CH2-1050 cm-1 wavenumber [15]. For the two acrylate monomers employed, lauryl methacrylate peaks around 2922 and 2851 cm-1 wavenumber are attributed to the methyl, CH3 and methylene, CH2 of its long chain and the absorption peak around 1100 cm-1 is due to the stretching vibration of C-O-C in the THFMA. Moreover, for both LMA and THFMA, the characteristic prominent peak around 1720 cm-1 is attributed to the carbonyl group (C=O).
The infrared spectra for the grafted starch samples showed a decrease in the absorption peak intensity corresponding to the hydroxyl group OH- and indicated the expected C=O absorption peak at 1731 cm-1 (Fig. 3(b)). These confirmed that the C=O came from grafted monomers, which verified the graft reaction had occurred between the native starch and grafting monomer and that higher monomer loading gave stronger intensities of the carbonyl peaks [16].
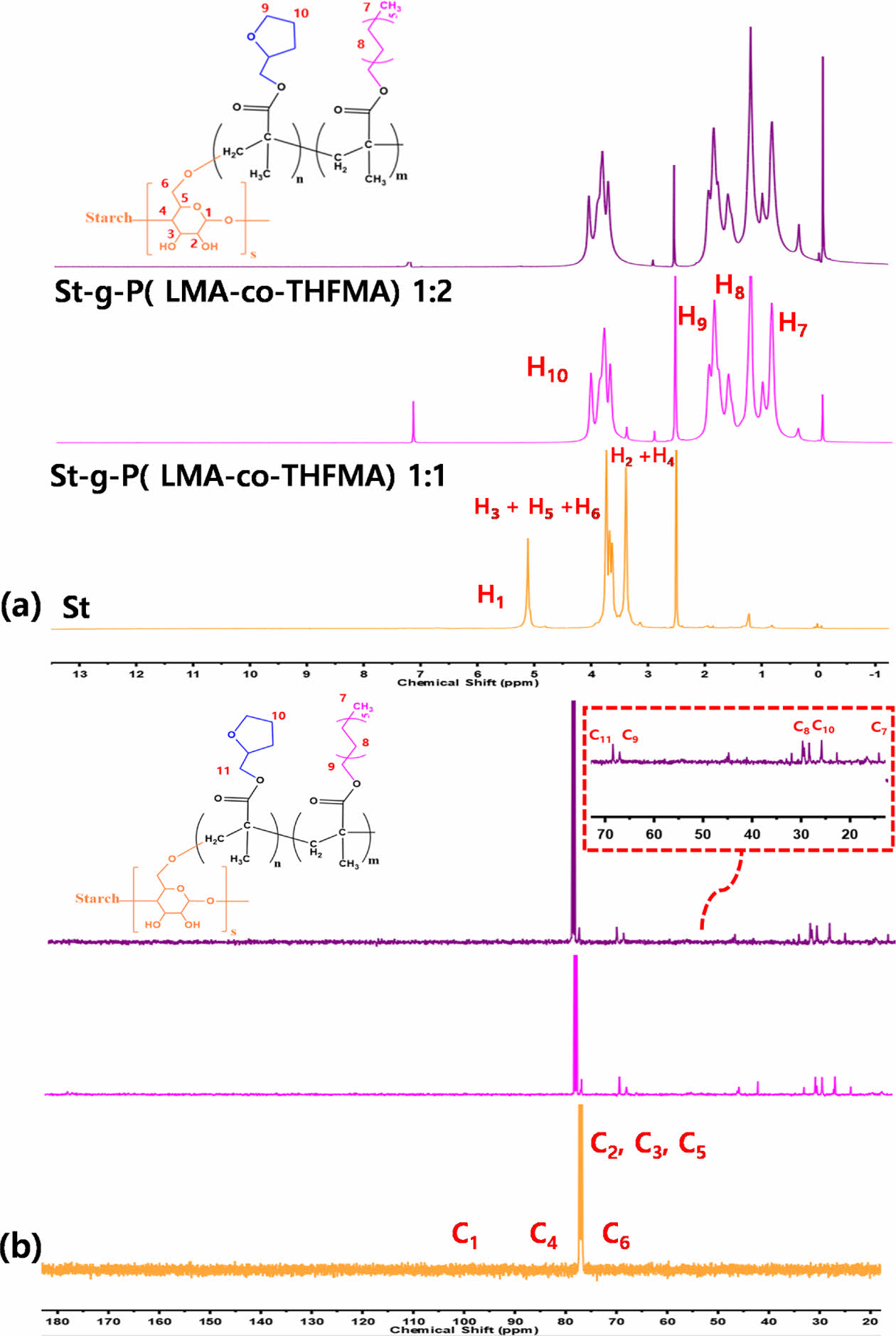
Besides FT-IR characterization, both 1H NMR and 13C NMR spectra were also performed to confirm the graft polymerization of starch (Fig. 4). The 1H NMR results are shown in Fig. 4(a). The chemical shifts between 5.0 and 6.0 ppm attributed to the vinyl protons of LMA and THFMA, thus are not observed in the 1H NMR spectrum of m-starch, confirming the grafting polymerization onto the starch backbone. The chemical shift at 1.25 (1-2) ppm corresponds to the methylene protons of LMA. The methine (CH) and methylene (CH2) protons in the tetrahydrofurfuryl ring and methylene protons near the ester bond in the THFMA and LMA show overlapping signals in the range of 1-4 ppm.
Moreover, as shown in Fig. 4(b), the 13C NMR spectra of m-starch exhibited a characteristic chemical shift between 20-70 ppm. The chemical shift at 29 ppm (7) is assigned to the carbon on the methylene group in the LMA long chain, whereas the 67 ppm peak (9) refers to the carbon on the long chain linked to the carboxyl group in the LMA. Moreover, the chemical shifts at 26 ppm (10) correspond to the carbon in the furan ring on the THFMA structure and 68 ppm (11) to the carbon linked to the ester group in THFMA [17]. Both 1H NMR and 13C NMR results verified the effective grafting of LMA and THFMA onto starch.
3.2 Surface Attributes of St-g-P(LMA-co-THFMA)
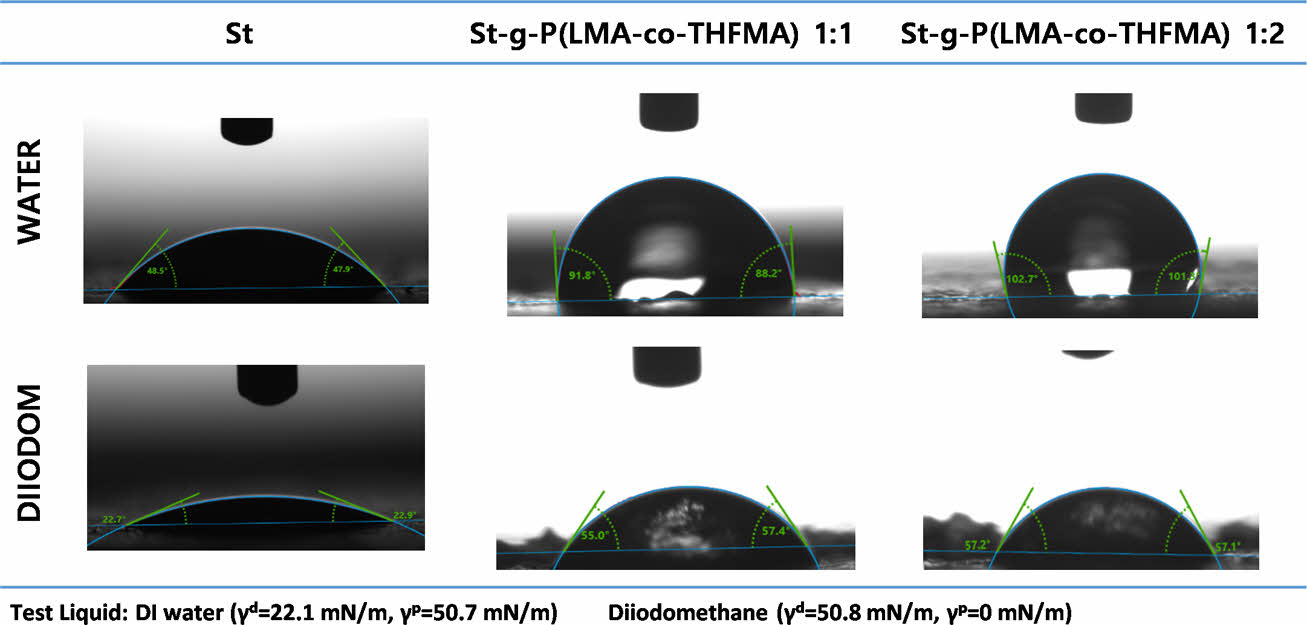
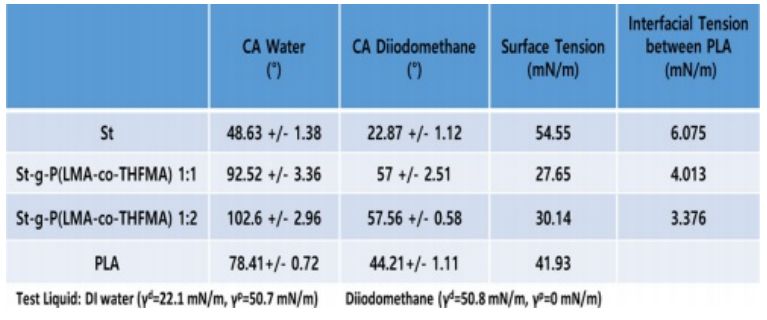
Contact angle measurements were performed to study the hydrophobicity of native starch and modified starch after the grafting synthesis. The contact angle of St and m-St were measured using two liquids, and the results are shown in Fig. 5. The water contact angle of starch of 48° (±1.38) exhibited the characteristic hydrophilic attribute of the native starch. The m-St 1:1 (w/w) ratio showed a water contact angle of 92° (±3.36) and a more hydrophobic ratio of 1:2 (w/w) with a contact angle of 102° (±2.96) which is attributed to the increased monomer acrylates weight ratio incorporation due to the long carbon chains of the LMA acrylate.
3.2 Morphology and Dispersion of St-g-P(LMA-co-THFMA) on PLA Films
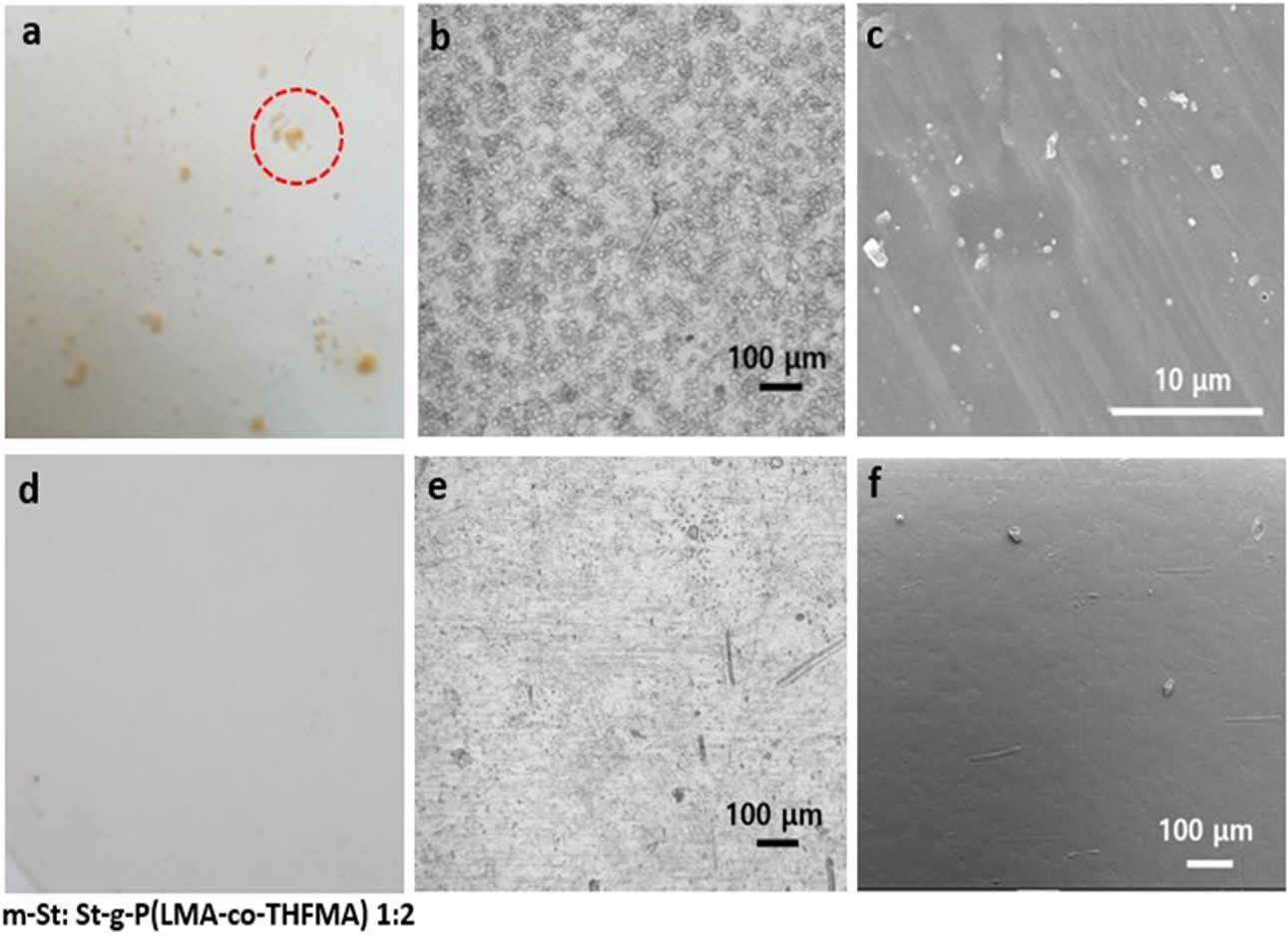
The native starch photo image shown in Fig. 6(a) clearly exhibited rough surfaces with irregular agglomerate starch particles on the PLA sample. On the contrary, modified starch on PLA revealed a smooth and compact surface (Fig. 6(d)). Upon optical images with higher magnification, evident native starch particles were detected in Fig. 6(b), thus poor interfacial compatibility between starch and PLA matrix was confirmed. In contrast, a clearly well-dispersed m-St within the PLA matrix was observed on Fig. 6(b) and (e). To corroborate these compatibility results, the interfacial tension between St and PLA was calculated using water and diiodomethane contact angle of St and m-St as listed in Table 2. Here, the interfacial tension between St and PLA matrix decreased when the acrylate monomer was grafted onto starch reporting interfacial tension values of γSt=6.075 mN/m, γm-St=4.013 mN/m, γm-St= 3.376 mN/m for St, m-St 1:1 and m-St 1:2, respectively.
Scanning electron microscopy (SEM) analyses also revealed the significant dissimilarity in starch surface morphology before and after grafting modification. Fig. 6(c) and (f) showed the SEM images of PLA/St 10% and PLA/m-St 10%, respectively. A significant difference was observed as the images still revealed the native starch microstructure on the PLA/St sample compared to the smoothly dispersed starch on PLA after monomer acrylates were grafted. This demonstrates that the structure undergoes significant changes during the graft copolymerization process.
3.4 Mechanical Properties of St-g-P(LMA-co-THFMA) in PLA
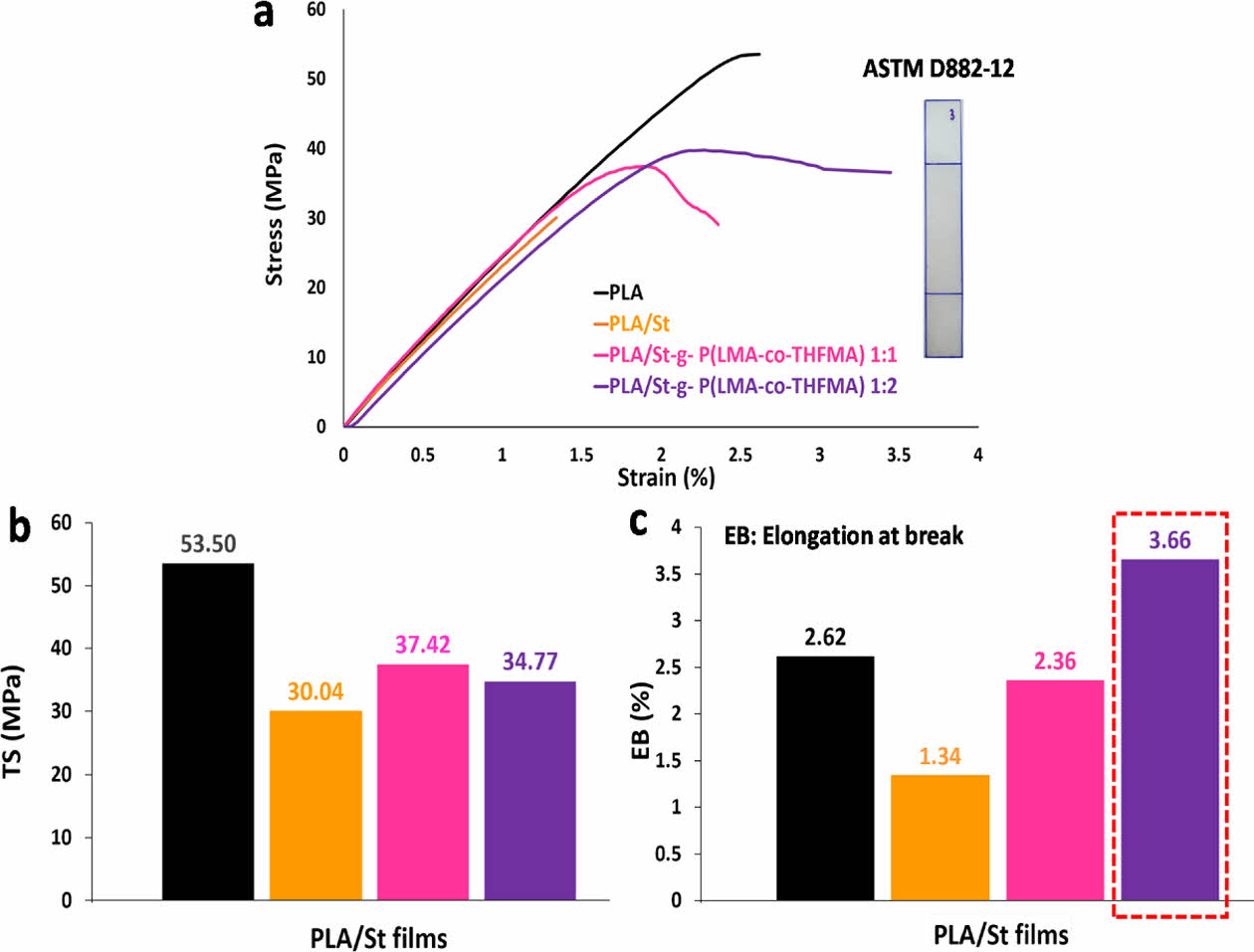
The mechanical properties of bulk PLA and PLA/St films were evaluated. The tensile strength and elongation at break of PLA, PLA/St, PLA-g-P(LMA-co-THFMA) 1:1, and PLA-g-P(LMA-co-THFMA) 1:2 composites are exhibited in Fig. 7(a)-(c).
The PLA showed a tensile strength of 53.5 MPa and elongation at break of 2.62%. Upon the addition of native starch, the tensile strength of PLA decreased to 30.04 MPa and elongation at break to 1.34%. The 10 wt% addition of (LMA-co-THFMA) 1:1 and (LMA-co-THFMA) 1:2 caused a gradual decrease of tensile strength to 37.42 and 34.77 MPa, respectively. However, the elongation at break of PLA-g-P(LMA-co-THFMA) 1:2 increased to 3.66% as shown in Fig. 7(c), due to a slight improvement in the interfacial compatibility. This is most likely due to the long carbon chains on LMA shown on the efficient m-St dispersion, thus good interfacial compatibility with PLA.
This was validated as shown on SEM images, which exhibited remarkable dispersion of m-St on PLA compared to the visual heterogenous starch particles responsible for the lack of interfacial compatibility with the PLA matrix. Consequently, the mechanical properties of PLA/St composites are attributed mainly to the improved interfacial interaction between m-St and PLA.
|
Fig. 2 The proposed initiation mechanism for grafting LMA-coTHFMA onto starch |
|
Fig. 3 FT-IR spectra of (a) native starch, LMA, THFMA, (b) St-g-P (LMA-co-THFMA) 1:1 and 1:2 (w/w) |
|
Fig. 4 (a) 1H NMR and (b) 13C NMR spectra of St and m-St |
|
Fig. 5 Native starch and m-St contact angle |
|
Fig. 6 PLA/St 10% (a) photo, (b) optical, (c) SEM image. PLA/mSt 10% (d) photo, (e) optical, (f) SEM image |
|
Fig. 7 (a) Stress-strain curves, (b) tensile strength (c) elongation at break for PLA, PLA/St, and PLA/m-St ratios |
In this work, an eco-friendly approach for the preparation of a PLA/starch film was presented. Starch was successfully grafted by copolymerizing two biobased acrylates (LMA and THFMA) used to enhance the toughening properties in PLA. With a 10% addition of m-St 1:2, the use of LMA and THFMA improved the elongation at break to 3.66%, decreased the tensile strength to 34.77 MPa, and allowed for the preparation of different materials by modifying the ratio of m-St on PLA. Such behaviour is related to the improved dispersion of m-St 1:2 on PLA, which was confirmed by SEM images showing enhanced compatibility between modified starch and PLA matrix. In conclusion, eco-friendly modified starch was successfully prepared and applied to enhance the toughening properties of PLA. Further studies of PLA/m-St films are in progress to explore its approach in packaging applications.
This work was supported by the Technology Innovation Program (20013794, Center for Composite Materials and Concurrent Design) funded by the Ministry of Trade, Industry & Energy (MOTIE, KOREA) and the Commercialization Promotion Agency for R&D Outcomes (COMPA) funded by the Ministry of Science and ICT (MSIT) [2022 COMPA-004].
- 1. Mansour, G., Zoumakia, M., Marinopouloub, A., Tzetzisc, D., Prevezanosb, M., and Raphaelidesb, S.N., “Characterization and Properties of Non-granular Thermoplastic Starch - Clay Biocomposite Films”, Carbohydrate Polymers, Vol. 245, 2020, 116829.
-

- 2. Jung, J., Kim, S., Kim, S., Park, J., and Lee, W., “Research on the Development of the Properties of PLA Composites for Automotive Interior Parts”, Composites Research, Vol. 24, No. 3, 2011, pp. 9-13.
-

- 3. Roh, J.U., and Lee, W.I., “Manufacture of Continuous Glass Fiber Reinforced Polylactic Acid (PLA) Composite and Its Properties”, Composites Research, Vol. 26, No. 4, 2013, pp. 230-234.
-

- 4. Yu, M., Zheng, Y., and Tian, J., “Study on the Biodegradability of Modified Starch/Polylactic Acid (PLA) Composite Materials”, Royal Society of Chemistry Advances, Vol. 10, 2020, 26298.
-

- 5. Momeni, S., Ghomi, R., Shakiba, M., Shafiei-Navid, S., Abdouss, M., Bigham, A., Khosravi, F., Ahmadi, Z., Faraji, M., Abdouss, H., and Ramakrishna, S.,“The Effect of Poly(ethylene glycol) Emulation on the Degradation of PLA/Starch Composites”, Polymers, Vol. 13, 2021, 1019.
-

- 6. Çankaya, N., “Synthesis of Graft Copolymers onto Starch and Its Semiconducting Properties”, Results in Physics, Vol. 6, 2016, pp. 538-542.
-

- 7. Weerapoprasit, C., and Prachayawarakorn, J., “Characterization and Properties of Biodegradable Thermoplastic Grafted Starch Films by Different Contents of Methacrylic Acid”, International Journal of Biological Macromolecules, Vol. 123, 2019, pp. 657-663.
-

- 8. Zuo, Y., He, X., Li, P., Li, W., and Wu, Y., “Preparation and Characterization of Hydrophobically Grafted Starches by In Situ Solid Phase Polymerization”, Polymers, Vol. 11, 2019, 72.
-

- 9. Bunkerd, R., Molloy, R., Somsunan, R., Punyodom, W., Topham, P., and Tighe, B., “Synthesis and Characterization of Chemically-Modified Cassava Starch Grafted with Poly(2-Ethylhexyl Acrylate) for Blending with Poly(Lactic Acid)”, Starch- Stärke, Vol. 70, 2018, 1800093.
-

- 10. Adorna, J., Aleman, C., Gonzaga, I., Pangasinan, J.,Sisican, K., Dang, V.,Doong, R., Ventura, R., and Ventura, J., “Effect of Lauric Acid on the Thermal and Mechanical Properties of Polyhydroxybutyrate (PHB)/Starch Composite Biofilms”, International Journal of Polymer Science, Vol. 2020, 2020, 7947109.
-

- 11. Sun, Y., Ma, Z., Xu, X., Liu, X., Liu, L., Huang, G., Liu, L., Wang, H., and Song, P., “Grafting Lignin with Bioderived Polyacrylates for Low-Cost, Ductile, and Fully Biobased Poly(lactic acid) Composites”, ACS Sustainable Chemistry & Engineering, Vol. 8, No. 5, 2020, pp. 2267-2276.
-

- 12. Kaur, K., Jindal, R., Maiti, M., and Mahajan, S., “Studies on the Properties and Biodegradability of PVA/Trapa Natans Starch (N-st) Composite Films and PVA/N-st-g-poly (EMA) Composite Films” International Journal of Biological Macromolecules, Vol. 123, 2019, pp. 826-836.
-

- 13. Chen, C., Tian, Y., Li, F., Hu, H., Wang, K., Kong, Z., Ying, W., Zhang, R., and Zhu, J., “Toughening Polylactic Acid by a Biobased Poly (Butylene 2,5-Furandicarboxylate)‑b‑Poly(Ethylene Glycol) Copolymer: Balanced Mechanical Properties and Potential Biodegradability”, Biomacromolecules, Vol. 22, 2021, pp. 374-385.
-

- 14. Zong, E., Liu, X., Liu, L., Wang, J., Song, P., Ma, Z., Ding, J., and Fu, S., “Graft Polymerization of Acrylic Monomers onto Lignin with CaCl2-H2O2 as Initiator: Preparation, Mechanism, Characterization, and Application in Poly(lactic acid)”, ACS Sustainable Chemistry & Engineering, Vol. 6, 2018, pp. 337-348.
-

- 15. Wang, L., Shen, J., Men, Y., Wu, Y., Peng, Q., Wang, X., Yang, R., Mahmood, K., and Liu, Z., “Corn Starch-based Graft Copolymers Prepared via ATRP at the Molecular Level”, Polymer Chemistry, Vol. 6, 2015, pp. 3480-3488.
-

- 16. Lu, C., Wang, C., Yu, J., Wang, J., and Chu, F., “Metal-free ATRP ‘Grafting from’ Technique for Renewable Cellulose Graft Copolymers”, Green Chemistry, Vol. 21, 2019, pp. 2759-2770.
-

- 17. Cazotti, J., Fritz, A., Garcia-Valdez, O., Smeets, N., Dubé, M., and Cunningham, M., “Grafting from Starch Nanoparticles with Synthetic Polymers via Nitroxide-Mediated Polymerization”, Macromolecular Rapid Communications, Vol. 40, 2019, 1800834.
-

 This Article
This Article
-
2022; 35(6): 419-424
Published on Dec 31, 2022
- 10.7234/composres.2022.35.6.419
- Received on Aug 8, 2022
- Revised on Sep 15, 2022
- Accepted on Nov 4, 2022
 Services
Services
- Abstract
1. introduction
2. materials and methods
3. results and discussion
4. conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Jonghwan Suhr
-
Department of Mechanical Engineering
- E-mail: suhr@skku.edu







 Copyright ⓒ The Korean Society for Composite Materials. All rights reserved.
Copyright ⓒ The Korean Society for Composite Materials. All rights reserved.