- Improving Gas Barrier Property of Polymer Based Nanocomposites Using Layer by Layer Deposition Method for Hydrogen Tank Liner
Suyeon Lee*, Hye Seong Han*, Dong Gi Seong*,**†
* School of Chemical Engineering, Pusan National University
** Department of Polymer Science and Engineering, Pusan National UniversityThis article is an open access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Owing to advantages of polymeric materials for hydrogen tank liner like light-weight property and high specific strength, polymer based composites have gained much attention. Despite of many benefits, polymeric materials for fuel cell tank cause problems which is critical to applications as low gas barrier property, and poor processability when adding fillers. For these reasons, improving gas barrier property of polymer composites is required to study for expanding application fields. This work presents impermeable polymer nanocomposites by introducing thin barrier coating using layer by layer (LBL) deposition method. Also, bi-layered and quad-layered nanocomposites were fabricated and compared for identifying relationship between deposition step and gas barrier property. Reduction in gas permeability was observed without interrupting mechanical property and processability. It is discussed that proper coating conditions were suggested when different coating materials and deposition steps were applied. We investigated morphology, gas barrier property and mechanical properties of fabricated nanocomposites by FE-SEM, Oxygen permeation analyzer, UTM, respectively. In addition, we revealed the mechanism of barrier performance of LBL coating using materials which have high aspect ratio.
Keywords: Hydrogen tank liner, Layer by layer coating, Gas barrier property, Nanocomposite
In recent years, hydrogen fuel cell electric vehicle (FCEV) has attracted much attention because its eco-friendly characteristics. The hydrogen energy systems have been suggested to reduce greenhouse gas and hazardous emissions from mobile vehicle sources. Especially, type 4 hydrogen tank, composed of carbon reinforced plastics and polymer composite, is much more prominent than other types owing to advantages of polymer composites [1,2]. Among the components of FCEV, liner is one of the main parts composing hydrogen tank, which prevent gas molecules from escaping of tank. In these days, polymer based composite has been used for liner material because of its lightweight and high impact strength, while conventional liners use heavy materials like metals. However, low gas barrier property is the biggest drawback of polymer materials, so numerous papers have been studied for make lower gas permeability of polymer composites [3-5].
Polyamide 6 (PA6), polyvinyl alcohol (PVA) and ethylene vinyl alcohol (EVOH) are polymers which have high gas barrier property because of its crystalline structure [6,7]. Although its structural strength, there are still limitations for higher hydrogen gas barrier property because hydrogen molecules are much smaller than other gas molecules. Therefore, blending with materials which have high barrier property is required for improving performance [8]. There are two main steps that effect to gas barrier mechanism of polymer materials. First, solubility coefficient can be identified when the gas molecules dissolve in the polymer and chemical similarity between polymer and gas makes permeability higher. Second, gas molecules diffuse in the polymer as gas concentration gradient in matrix. Also, tortuous path gets longer when the fillers which has high aspect ratio combine with polymer materials. It is known that longer tortuous path makes gas permeability lower because it takes more time to diffuse. Because of small size of hydrogen molecules, it is more difficult that fabricating composites which have high hydrogen gas barrier property [9].
Nano-sized particles like graphene, montmorillonite (MMT) were widely used fillers for reinforcing gas barrier properties of polymer materials because of its plate shape. Mixing these particles can attribute to longer tortuous path of gas molecules, which is the main mechanism of barrier property [10-12]. However, simple mixing of polymer and nano fillers can cause serious problems as aggregation of particles, decreasing processability and mechanical strength. Among many problems, poor processability of composite is the most critical for industry applications. Injection molding and blow molding methods are representative process for fabricating hydrogen tank liners, which means that materials have to maintain elongational strain and mechanical strength. Accordingly, it is necessary that fabricating polymer composites without any decreasing processability and mechanical performances. Thus, many studies have been researched coating on polymer substrate to improve various properties.
Layer by layer (LBL) deposition method is a kind of coating process to manufacture nanocomposites. It has been researched for improving various performances with a view to applications since the early 1990s [13-16]. Electrostatic attraction of polymer electrolytes and charged molecules are the mechanisms of multilayered nanocomposite by LBL method. With LBL process, thin films having high performance can be fabricated with a very low concentration of solutions. Among various coating solutions, chitosan, MMT, branched polyethyleneimine (BPEI) and polyacrylic acid (PAA) are explored to fabricate functional nanocomposite. Chitosan and BPEI have positive charge, PAA and MMT have negative charge for deposition method [17-19]. Nanocomposites which have thin coating thickness and dense structure can be fabricated without interrupting processability and mechanical properties by LBL deposition process. Moreover, weight reduction efficiency of polymer composites still maintains when ceramic material like MMT is introduced on substrate, because ultra-thin coating is fabricated by LBL method and low concentration of coating solution is used for this process.
Spray coating, dip coating and vacuum assisted filtration method were commonly used process for LBL coating. Most of all, dip coating process was conducted for LBL deposition since it is one of the most widely used and simple coating method [20-23].
In this study, we aim to fabricate nanocomposites with superior gas barrier property using LBL method and maintaining high processability of polymer materials. In addition, bi-layer (BL) and quad-layer (QL) nanocomposites were compared, and we are trying to find proper conditions by applying different coating materials. Morphology, gas permeability and mechanical properties were investigated performance of nanocomposites. Owing to difficulty of estimating hydrogen gas permeability, oxygen gas was used instead of hydrogen gas because it has similar polarity with hydrogen. This work demonstrates the gas barrier efficiency of nanocoating by LBL method with various coating materials for industrial fields applications.
2.1 Materials
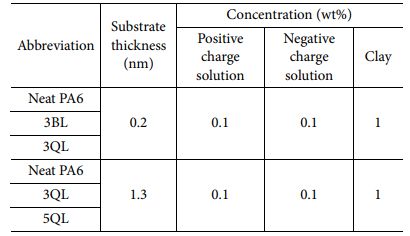
Commercially available materials of chitosan, BPEI, PAA and MMT were used for experiments and all chemicals were used as received. Branched polyethyleneimine (BPEI) (Average Mw ~10,000, Sigma Aldrich, USA), Polyacrylic acid (PAA) solution (Average Mw ~100,000, 35 wt% in H2O, Sigma Aldrich, USA) and Chitosan (CH) (low molecular weight, Sigma Aldrich, USA) were used to prepare 0.1 wt% solutions and Montmorillonite (MMT) (K 10, powder, Sigma Aldrich, USA) was used to prepare 1 wt% solution, respectively. All chemicals used in this study were immersed and dispersed in D.I. water. A commercial PA6 was kindly supported by Hyundai Motors and manufactured by compression molding to equip disc shape for investigating permeability at 270°C, 30 min. Fabricated PA6 films were utilized as a substrate with 0.2 mm and 1.3 mm of thickness and dried before exploring experiments.
2.2 Fabrication of Bi-layer (BL) Composite
Bi-layered nanocomposites coated by LBL deposition process were fabricated using CH and MMT, positive charged and negative charged, respectively. A 0.1 wt% aqueous solutions of CH and MMT were easily produced and stirred at 300 rpm for overnight to dispersed uniformly. At first, polymer substrate was dipped into each solution alternatively for 5 min and when 1BL was formed, dipping time decreased to 1 min. Between processes, rinse and dry step was required to eliminate excessive coating solutions and moisture. LBL coated nanocomposites were fabricated by repeating steps 3 times (3BL) and 5 times (5BL).
2.3 Fabrication of Quad-layer (QL) Composite
Quad-layered nanocomposites were fabricated by the same LBL process with BL coated specimens. To identifying differences by layer deposition numbers and materials, quad-layered composites were compared with bi-layered ones. Dipping time, concentration of solutions and step sequence were the same. As a results, 3QL and 5QL composites were fabricated using BPEI, PAA and MMT solutions. Illustration images of preparation was indicated in Fig. 1 and composition of specimens in this study was shown in Table 1.
2.4 Characterizations
Fourier transform infrared (FT-IR) (FT-IR-4600LE, JASCO, Japan) were investigated by attenuated total reflectance (ATR) mode to confirm MMT coating on substrate. Morphology of nanocomposites was characterized by a Field emission Scanning Electron Microscope (FE-SEM) (JSM-7610F, JEOL, Japan), (VEGA II LSU, TESCAN, Czech Republic) and Oxygen permeation analyzer (OX-TRAN 2/21, ML, MOCON, USA) was used to determine oxygen gas permeability. For mechanical testing, ASTM D3039 was used to characterize tensile properties of fabricated nanocomposites by Universal testing machine (UTM) (Instron 5985, Instron, USA). The shape of specimens has a rectangular cross section with dog-bone shape. In addition, to confirm stability as used in hydrogen tank field, we performed tensile test at various temperature.

|
Fig. 1 Schematic figure of quad-layered nanocomposite fabrication through Layer by layer (LBL) deposition method |
3.1 Identification of LBL Coating
Polymer composites with thin nano-sized coating by LBL depostion method were fabricated using dip coating process. The FT-IR spectra of neat PA6 film and LBL coated composites are compared in Fig. 2(a) and (b). Peaks at 1540 cm-1 and 2800-2950 cm-1 correspond to N-H and C-H bond which are the typical chemical bonding of PA6. Peaks at 918 cm-1 and 1000-1150 cm-1 correspond to Al-OH and Si-O bond, which means that LBL coating was successfully conducted on substrate.
Also, morphology analysis of both nanocomposites were characterized by FE-SEM, indicating in Fig. 3. It is confirmed that LBL coating was perfectly carried out on substrate, resulted in bi-layered nanocomposites with MMT particles, while neat pa6 has clean and smooth surface (Fig. 3(a)-(c)) Aggregation of MMT particles by increasing BL numbers was observed through growing size. However, in case of QL nanocomposites, more dense structure of nanocoating was confirmed at 5QL than 3QL specimen, which was indicated in Fig. 3(d)-(f).
3.2 Gas Barrier Property of LBL Coated Composites
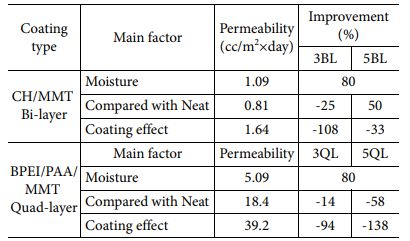
We carried out oxygen gas permeability test to investigate barrier properties of fabricated nanocomposites. In this study, it is expected that the proper condition of enhancing gas barrier property can be differentiated by deposition systems and coating materials. In Fig. 4(a), increasing permeability was observed when the substrate exposure to moisture because of hydrophilic characteristics of PA6. Moisture acts like a plasticizer when the hydrophilic materials were exposed to water. On the other hand, 3BL nanocomposite has much lower oxygen permeability although LBL process was implemented in water condition, which means that LBL coating is effective for barrier properties. Oxygen permeability slightly increased when the BL number was increased, resulted from aggregation of particles which was indicated in morphology analysis. Oxygen transmittance rate of quad-layered nanocomposites is performed as shown in Fig. 4(b). In case of QL specimens, each LBL coated samples were compared with their own neat samples, because there was huge difference between uncoated specimens owing to thick thickness of substrate. Increasing permeability was observed in neat PA6 after water exposure, as previously mentioned. Tremendous improvement in barrier property was achieved at 5QL nanocomposite, decreased 58% in comparison with neat sample. However, barrier property of 3QL was enhanced slightly because of intrinsic hydrophilic characteristic of substrate. The coating efficiency parameters by coating type and main factors are shown in Table 2. We evaluate the gas barrier property with three main factors which is moisture, neat sample and coating effect by itself. 5QL nanocomposite has more higher barrier efficiency than 5BL because QL consist of denser structure and the free volume of chitosan in BL increases by moisture. Increasing free volume of chitosan is critical to gas barrier property since free volume act as an amorphous region which can be penetrated by gas molecules.
3.3 Mechanical Properties of Nanocomposites
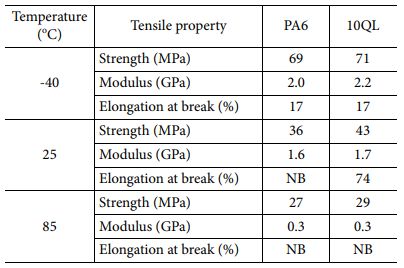
To identifying additional improvement of mechanical properties, 10QL coated nanocomposite was compared with neat PA6 film at different temperatures. Differences of performance at working temperature should be investigated because of the materials for hydrogen tank require to maintain mechanical property as the gas inside tank continue to expand and shrink. Fig. 5 shows tensile properties of uncoated and LBL coated nanocomposites. Tensile strength and modulus increase by decreasing temperature meanwhile tensile strain decreases because polymer material became more brittle in cryogenic condition, as shown in Table 3. In case of LBL coated nanocomposites, tensile strength increases 20% (36 MPa to 43 MPa) since LBL coating includes MMT whose mechanical property was higher than substrate, which means that LBL coating strengthens composites. Also, this phenomenon was observed at all working temperatures. It is indicated that LBL coating improves mechanical property of composites at various circumstances without hindering processability.
3.4 Mechanism of LBL Coating for Barrier Property
Schematic figure of gas barrier mechanism of LBL coated nanocomposites was indicated in Fig. 6. When the polymer materials exposed to hydrogen gas atmosphere, gas molecules easily penetrate through amorphous region of matrix. However, coated nanocomposites with materials which have high aspect ratio can make tortuous path longer. Higher tortuosity means lower gas permeability since it is complicated to pass through the materials. In addition, gas permeation in the polymer is influenced by amount of amorphous and crystalline structures in the polymer, backbone orientation and side chains [3]. Fillers like MMT play as a crystalline structure which are more effective as permeation barriers. Therefore, much lower hydrogen gas permeability of LBL coated nanocomposites was achieved.

|
Fig. 2 FT-IR spectra of (a) Neat PA6 film and (b) LBL coated nanocomposite |

|
Fig. 3 Morphological observation of bi-layered nanocomposites at 250x; (a) neat PA6, (b) 3BL and (c) 5BL, and quad-layered nanocomposites at same magnification; (d) neat PA6, (e) 3QL and (f) 5QL |

|
Fig. 4 Oxygen gas permeability of (a) bi-layered and (b) quadlayered nanocomposites before and after LBL coating process |

|
Fig. 5 Tensile properties of (a) neat PA6 film and (b) 10QL coated nanocomposites at different temperature and (c) mechanical property comparison of all specimens |

|
Fig. 6 Illustration images of gas barrier mechanism by LBL coated nanocomposites |
|
Table 2 Oxygen permeability of nanocomposites by different LBL system compared with various parameters for barrier property |
In this work, we successfully fabricated LBL coated nanocomposite with different deposition steps. Ultra-thin film is successfully coated on PA6 substrate through LBL method, which was characterized by FT-IR and FE-SEM. It is observed that gas permeability decreases when LBL coating was conducted on the substrate. Indeed, we suggest a proper deposition numbers with different layer composition for gas barrier property. It is indicated that 5QL nanocomposite is more effective than 5BL because the free volume of chitosan in BL increase when exposed to water, and more dense structure can be fabricated by QL system. In addition, improvement of mechanical properties was found by LBL coating with 10QL of layer numbers. Our investigations show that LBL coating including organic and inorganic materials make composite stronger and coated nanocomposites have high gas barrier property at the same time. Moreover, with LBL coating, improvement of flame retardant property can be expected because BPEI and PAA form char after combustion, which contribute to expand application fields of this nanocomposites. From this work, it is provided that a proper condition by LBL coating can be suggested with various coating materials and it can be used as a standard for applying industrial applications.
This work was supported by a 2-Year Research Grant of Pusan National University.
- 1. Su, Y., Lv, H., Zhou, W., and Zhang, C., “Review of the Hydrogen Permeability of the Liner Material of Type IV On-Board Hydrogen Storage Tank,” World Electric Vehicle Journal, Vol. 12, No. 3, 2021, pp. 130.
-

- 2. Mori, D., and Hirose, K., “Recent Challenges of Hydrogen Storage Technologies for Fuel Cell Vehicles,” International Journal of Hydrogen Energy, Vol. 34, No. 10, 2009, pp. 4569-4574.
-

- 3. Ichikawa, T., Hanada, N., Isobe, S., Leng, H., and Fujii, H., “Composite Materials Based on Light Elements for Hydrogen Storage,” Materials Transactions, Vol. 46, No. 1, 2005, pp. 1-14.
-

- 4. Thompson, L., Hydrogen, Fuel Cells, and Infrastructure Technologies FY 2002 Progress Report. IV.C, Vol. 6, 2005.
- 5. Khalil, Y., Newhouse, N., Simmons, K., and Dedrick, D., “Potential Diffusion-based Failure Modes of Hydrogen Storage Vessels for On-board Vehicular Use”, 2010.
- 6. Hu, Y., Prattipati, V., Mehta, S., Schiraldi, D., Hiltner, A., and Baer, E., “Improving Gas Barrier of PET by Blending with Aromatic Polyamides,” Polymer, Vol. 46, No. 8, 2005, pp. 2685-2698.
-

- 7. Maes, C., Luyten, W., Herremans, G., Peeters, R., Carleer, R., and Buntinx, M., “Recent Updates on the Barrier Properties of Ethylene Vinyl Alcohol Copolymer (EVOH): A Review,” Polymer Reviews, Vol. 58, No. 2, 2018, pp. 209-246.
-

- 8. Picard, E., Vermogen, A., Gérard, J., and Espuche, E., “Barrier Properties of Nylon 6-montmorillonite Nanocomposite Membranes Prepared by Melt Blending: Influence of the Clay Content and Dispersion State: Consequences on Modelling,” Journal of Membrane Science, Vol. 292, No. 1-2, 2007, pp. 133-144.
-

- 9. Cui, Y., Kumar, S., Kona, B.R., and van Houcke, D., “Gas Barrier Properties of Polymer/clay Nanocomposites,” RSC Advances, Vol. 5, No. 78, 2015, pp. 63669-63690.
-

- 10. Idris, A., Muntean, A., and Mesic, B., “A Review on Predictive Tortuosity Models for Composite Films in Gas Barrier Applications,” Journal of Coatings Technology and Research, Vol. 19, No. 3, 2022, pp. 1-18.
-

- 11. Feldman, D., “Polymer Nanocomposite Barriers,” Journal of Macromolecular Science, Part A, Vol. 50, No. 4, 2013, pp. 441-448.
-

- 12. Dhieb, F.B., Dil, E.J., Tabatabaei, S.H., Mighri, F., and Ajji, A., “Effect of Nanoclay Orientation on Oxygen Barrier Properties of LbL Nanocomposite Coated Films,” RSC Advances, Vol. 9, No. 3, 2019, pp. 1632-1641.
-

- 13. Caruso, F., Caruso, R.A., and Mohwald, H., “Nanoengineering of Inorganic and Hybrid Hollow Spheres by Colloidal Templating,” Science, Vol. 282, No. 5391, 1998, pp. 1111-1114.
-

- 14. Decher, G., and Schlenoff, J.B., “Multilayer Thin Films: Sequential Assembly of Nanocomposite Materials”, John Wiley & Sons, 2006.
- 15. Donath, E., Sukhorukov, G.B., Caruso, F., Davis, S.A., and Möhwald, H., “Novel Hollow Polymer Shells by Colloid‐templated Assembly of Polyelectrolytes,” Angewandte Chemie International Edition, Vol. 37, No. 16, 1998, pp. 2201-2205.
-

- 16. Sukhorukov, G.B., Donath, E., Lichtenfeld, H., Knippel, E., Knippel, M., Budde, A., and Möhwald, H., “Layer-by-layer Self Assembly of Polyelectrolytes on Colloidal Particles,” Colloids Surf Physicochem Eng Aspects, Vol. 137, No. 1-3, 1998, pp. 253-266.
-

- 17. Laufer, G., Kirkland, C., Cain, A.A., and Grunlan, J.C., “Clay–chitosan Nanobrick Walls: Completely Renewable Gas Barrier and Flame-retardant Nanocoatings,” ACS Applied Materials & Interfaces, Vol. 4, No. 3, 2012, pp. 1643-1649.
-

- 18. Lazar, S., Garcia‐Valdez, O., Kennedy, E., Champagne, P., Cunningham, M., and Grunlan, J., “Crosslinkable‐chitosan‐enabled Moisture‐resistant Multilayer Gas Barrier Thin Film,” Macromolecular Rapid Communications, Vol. 40, No. 6, 2019, pp. 1800853.
-

- 19. Tzeng, P., Lugo, E.L., Mai, G.D., Wilhite, B.A., and Grunlan, J.C., “Super Hydrogen and Helium Barrier With Polyelectolyte Nanobrick Wall Thin Film,” Macromolecular Rapid Communications, Vol. 36, No. 1, 2015, pp. 96-101.
-

- 20. Lee, S., Seong, D., Ju, Y., Kwak, H.W., Kim, W.S., and Lee, D., “Revealing the Flame Retardancy Mechanism of Highly Transparent Cellulose Nanopapers Fabricated by Vacuum Filtration Assisted Layer-by-layer Deposition,” Carbohydrate Polymers, Vol. 246, 2020, pp. 116628.
-

- 21. Beneventi, D., Zeno, E., and Chaussy, D., “Rapid Nanopaper Production by Spray Deposition of Concentrated Microfibrillated Cellulose Slurries,” Industrial Crops and Products, Vol. 72, 2015, pp. 200-205.
-

- 22. Bertrand, P., Jonas, A., Laschewsky, A., and Legras, R., “Ultrathin Polymer Coatings by Complexation of Polyelectrolytes at Interfaces: Suitable Materials, Structure and Properties,” Macromolecular Rapid Communications, Vol. 21, No. 7, 2000, pp. 319-348.
-

- 23. Mautner, A., Lee, K., Tammelin, T., Mathew, A.P., Nedoma, A.J., Li, K., and Bismarck, A., “Cellulose Nanopapers as Tight Aqueous Ultra-filtration Membranes,” Reactive and Functional Polymers, Vol. 86, 2015, pp. 209-214.
-

 This Article
This Article
-
2022; 35(3): 121-126
Published on Jun 30, 2022
- 10.7234/composres.2022.35.3.121
- Received on Jun 3, 2022
- Revised on Jun 15, 2022
- Accepted on Jun 17, 2022
 Services
Services
- Abstract
1. introduction
2. experimental
3. results and discussion
4. conclusions
- Acknowledgements
- References
- Full Text PDF
Shared
 Correspondence to
Correspondence to
- Dong Gi Seong
-
* School of Chemical Engineering, Pusan National University
** Department of Polymer Science and Engineering, Pusan National University - E-mail: dgseong@pusan.ac.kr






 Copyright ⓒ The Korean Society for Composite Materials. All rights reserved.
Copyright ⓒ The Korean Society for Composite Materials. All rights reserved.